Mechanism of oral tolerance induction to therapeutic proteins
- PMID: 23123293
- PMCID: PMC3578149
- DOI: 10.1016/j.addr.2012.10.013
Mechanism of oral tolerance induction to therapeutic proteins
Abstract
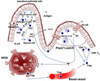
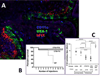
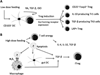
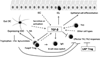
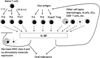
Oral tolerance is defined as the specific suppression of humoral and/or cellular immune responses to an antigen by administration of the same antigen through the oral route. Due to its absence of toxicity, easy administration, and antigen specificity, oral tolerance is a very attractive approach to prevent unwanted immune responses that cause a variety of diseases or that complicate treatment of a disease. Many researchers have induced oral tolerance to efficiently treat autoimmune and inflammatory diseases in different animal models. However, clinical trials yielded limited success. Thus, understanding the mechanisms of oral tolerance induction to therapeutic proteins is critical for paving the way for clinical development of oral tolerance protocols. This review will summarize progress on understanding the major underlying tolerance mechanisms and contributors, including antigen presenting cells, regulatory T cells, cytokines, and signaling pathways. Potential applications, examples for therapeutic proteins and disease targets, and recent developments in delivery methods are discussed.
Copyright © 2012 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Induction of ovalbumin-specific tolerance by oral administration of Lactococcus lactis secreting ovalbumin.Gastroenterology. 2007 Aug;133(2):517-28. doi: 10.1053/j.gastro.2007.04.073. Epub 2007 May 3. Gastroenterology. 2007. PMID: 17681173
-
Oral administration of type-II collagen peptide 250-270 suppresses specific cellular and humoral immune response in collagen-induced arthritis.Clin Immunol. 2007 Jan;122(1):75-84. doi: 10.1016/j.clim.2006.08.004. Epub 2006 Oct 11. Clin Immunol. 2007. PMID: 17045846
-
Mechanisms of Oral Tolerance.Pediatr Clin North Am. 2015 Dec;62(6):1523-9. doi: 10.1016/j.pcl.2015.07.013. Epub 2015 Sep 7. Pediatr Clin North Am. 2015. PMID: 26456448 Free PMC article. Review.
-
Co-administration of CD40 agonistic antibody and antigen fails to overcome the induction of oral tolerance.Immunology. 2004 Jan;111(1):19-26. doi: 10.1111/j.1365-2567.2004.01787.x. Immunology. 2004. PMID: 14678195 Free PMC article.
-
Oral tolerance to prevent anti-drug antibody formation in protein replacement therapies.Cell Immunol. 2022 Dec;382:104641. doi: 10.1016/j.cellimm.2022.104641. Epub 2022 Nov 14. Cell Immunol. 2022. PMID: 36402002 Free PMC article. Review.
Cited by
-
Edible plants for oral delivery of biopharmaceuticals.Br J Clin Pharmacol. 2017 Jan;83(1):71-81. doi: 10.1111/bcp.12949. Epub 2016 May 9. Br J Clin Pharmacol. 2017. PMID: 27037892 Free PMC article. Review.
-
Multidimensional futuristic approaches to address the pandemics beyond COVID-19.Heliyon. 2023 Jun;9(6):e17148. doi: 10.1016/j.heliyon.2023.e17148. Epub 2023 Jun 11. Heliyon. 2023. PMID: 37325452 Free PMC article. Review.
-
Poor Lymphocyte Infiltration to Primary Tumors in Acral Lentiginous Melanoma and Mucosal Melanoma Compared to Cutaneous Melanoma.Front Oncol. 2020 Dec 17;10:524700. doi: 10.3389/fonc.2020.524700. eCollection 2020. Front Oncol. 2020. PMID: 33392063 Free PMC article.
-
Glucocorticoid-Induced TNF Receptor Family-Related Protein Ligand is Requisite for Optimal Functioning of Regulatory CD4(+) T Cells.Front Immunol. 2014 Feb 3;5:35. doi: 10.3389/fimmu.2014.00035. eCollection 2014. Front Immunol. 2014. PMID: 24550919 Free PMC article.
-
Vaccination via Chloroplast Genetics: Affordable Protein Drugs for the Prevention and Treatment of Inherited or Infectious Human Diseases.Annu Rev Genet. 2016 Nov 23;50:595-618. doi: 10.1146/annurev-genet-120215-035349. Epub 2016 Oct 21. Annu Rev Genet. 2016. PMID: 27893966 Free PMC article. Review.
References
-
- Moog F. The lining of the small intestine. Sci Am. 1981;245:154–158. 160, 162 et passiom. - PubMed
-
- Brandtzaeg P. Development and basic mechanisms of human gut immunity. Nutr Rev. 1998;56:S5–S18. - PubMed
-
- Macfarlane GT, Macfarlane S. Human colonic microbiota: ecology, physiology and metabolic potential of intestinal bacteria. Scand J Gastroenterol Suppl. 1997;222:3–9. - PubMed
-
- du Pre MF, Samsom JN. Adaptive T-cell responses regulating oral tolerance to protein antigen. Allergy. 2011;66:478–490. - PubMed
-
- Matzinger P, Kamala T. Tissue-based class control: the other side of tolerance. Nat Rev Immunol. 2011;11:221–230. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

