Protease-activated receptor-1 modulates hippocampal memory formation and synaptic plasticity
- PMID: 23113835
- PMCID: PMC3518655
- DOI: 10.1111/jnc.12075
Protease-activated receptor-1 modulates hippocampal memory formation and synaptic plasticity
Abstract
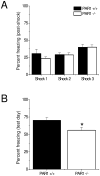
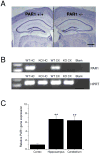
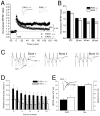
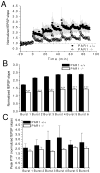
Protease-activated receptor-1 (PAR1) is an unusual G-protein coupled receptor (GPCR) that is activated through proteolytic cleavage by extracellular serine proteases. Although previous work has shown that inhibiting PAR1 activation is neuroprotective in models of ischemia, traumatic injury, and neurotoxicity, surprisingly little is known about PAR1's contribution to normal brain function. Here, we used PAR1-/- mice to investigate the contribution of PAR1 function to memory formation and synaptic function. We demonstrate that PAR1-/- mice have deficits in hippocampus-dependent memory. We also show that while PAR1-/- mice have normal baseline synaptic transmission at Schaffer collateral-CA1 synapses, they exhibit severe deficits in N-methyl-d-aspartate receptor (NMDAR)-dependent long-term potentiation (LTP). Mounting evidence indicates that activation of PAR1 leads to potentiation of NMDAR-mediated responses in CA1 pyramidal cells. Taken together, this evidence and our data suggest an important role for PAR1 function in NMDAR-dependent processes subserving memory formation and synaptic plasticity.
© 2012 International Society for Neurochemistry.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Rapid plasticity at inhibitory and excitatory synapses in the hippocampus induced by ictal epileptiform discharges.Eur J Neurosci. 2009 Mar;29(6):1153-64. doi: 10.1111/j.1460-9568.2009.06663.x. Eur J Neurosci. 2009. PMID: 19302151
-
Dysbindin-1 loss compromises NMDAR-dependent synaptic plasticity and contextual fear conditioning.Hippocampus. 2014 Feb;24(2):204-13. doi: 10.1002/hipo.22215. Epub 2013 Nov 12. Hippocampus. 2014. PMID: 24446171 Free PMC article.
-
Developmental switch in requirement for PKA RIIbeta in NMDA-receptor-dependent synaptic plasticity at Schaffer collateral to CA1 pyramidal cell synapses.Neuropharmacology. 2009 Jan;56(1):56-65. doi: 10.1016/j.neuropharm.2008.08.013. Epub 2008 Aug 22. Neuropharmacology. 2009. PMID: 18789341 Free PMC article.
-
Recruitment of N-Type Ca(2+) channels during LTP enhances low release efficacy of hippocampal CA1 perforant path synapses.Neuron. 2009 Aug 13;63(3):372-85. doi: 10.1016/j.neuron.2009.07.013. Neuron. 2009. PMID: 19679076 Free PMC article.
-
Presynaptic residual calcium and synaptic facilitation at hippocampal synapses of mice with altered expression of SNAP-25.Brain Res. 2012 Jan 11;1431:1-12. doi: 10.1016/j.brainres.2011.10.035. Epub 2011 Oct 29. Brain Res. 2012. PMID: 22119397 Free PMC article.
Cited by
-
Protease Activated Receptor 2 (PAR2) Induces Long-Term Depression in the Hippocampus through Transient Receptor Potential Vanilloid 4 (TRPV4).Front Mol Neurosci. 2017 Mar 2;10:42. doi: 10.3389/fnmol.2017.00042. eCollection 2017. Front Mol Neurosci. 2017. PMID: 28303089 Free PMC article.
-
Emerging Roles of Protease-Activated Receptors (PARs) in the Modulation of Synaptic Transmission and Plasticity.Int J Mol Sci. 2021 Jan 16;22(2):869. doi: 10.3390/ijms22020869. Int J Mol Sci. 2021. PMID: 33467143 Free PMC article. Review.
-
Channel-mediated astrocytic glutamate modulates hippocampal synaptic plasticity by activating postsynaptic NMDA receptors.Mol Brain. 2015 Feb 3;8:7. doi: 10.1186/s13041-015-0097-y. Mol Brain. 2015. PMID: 25645137 Free PMC article.
-
Post Stroke Seizures and Epilepsy: From Proteases to Maladaptive Plasticity.Front Cell Neurosci. 2019 Sep 13;13:397. doi: 10.3389/fncel.2019.00397. eCollection 2019. Front Cell Neurosci. 2019. PMID: 31607864 Free PMC article. Review.
-
Novel pharmaceutical treatments for minimal traumatic brain injury and evaluation of animal models and methodologies supporting their development.J Neurosci Methods. 2016 Oct 15;272:69-76. doi: 10.1016/j.jneumeth.2016.02.002. Epub 2016 Feb 8. J Neurosci Methods. 2016. PMID: 26868733 Free PMC article. Review.
References
-
- Agulhon C, Fiacco TA, McCarthy KD. Hippocampal short- and long-term plasticity are not modulated by astrocyte Ca2+ signaling. Science. 2010;327:1250–4. - PubMed
-
- Arai A, Lynch G. Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Research. 1992;598:173–184. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

