Molecular basis for membrane pore formation by Bax protein carboxyl terminus
- PMID: 23110300
- PMCID: PMC4537061
- DOI: 10.1021/bi301195f
Molecular basis for membrane pore formation by Bax protein carboxyl terminus
Abstract
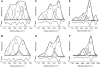
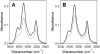
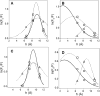
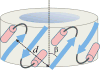
Bax protein plays a key role in mitochondrial membrane permeabilization and cytochrome c release upon apoptosis. Our recent data have indicated that the 20-residue C-terminal peptide of Bax (BaxC-KK; VTIFVAGVLTASLTIWKKMG), when expressed intracellularly, translocates to the mitochondria and exerts lethal effect on cancer cells. Moreover, the BaxC-KK peptide, as well as two mutants where the two lysines are replaced with glutamate (BaxC-EE) or leucine (BaxC-LL), have been shown to form relatively large pores in lipid membranes, composed of up to eight peptide molecules per pore. Here the pore structure is analyzed by polarized Fourier transform infrared, circular dichroism, and fluorescence experiments on the peptides reconstituted in phospholipid membranes. The peptides assume an α/β-type secondary structure within membranes. Both β-strands and α-helices are significantly (by 30-60 deg) tilted relative to the membrane normal. The tryptophan residue embeds into zwitterionic membranes at 8-9 Å from the membrane center. The membrane anionic charge causes a deeper insertion of tryptophan for BaxC-KK and BaxC-LL but not for BaxC-EE. Combined with the pore stoichiometry determined earlier, these structural constraints allow construction of a model of the pore where eight peptide molecules form an "α/β-ring" structure within the membrane. These results identify a strong membranotropic activity of Bax C-terminus and propose a new mechanism by which peptides can efficiently perforate cell membranes. Knowledge on the pore forming mechanism of the peptide may facilitate development of peptide-based therapies to kill cancer or other detrimental cells such as bacteria or fungi.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



Similar articles
-
Transmembrane pore formation by the carboxyl terminus of Bax protein.Biochim Biophys Acta. 2013 Feb;1828(2):732-42. doi: 10.1016/j.bbamem.2012.08.006. Epub 2012 Aug 18. Biochim Biophys Acta. 2013. PMID: 22906710
-
Peptides corresponding to helices 5 and 6 of Bax can independently form large lipid pores.FEBS J. 2006 Mar;273(5):971-81. doi: 10.1111/j.1742-4658.2006.05123.x. FEBS J. 2006. PMID: 16478471
-
The Bax BH3 peptide H2-H3 promotes apoptosis by inhibiting Bcl-2's pore-forming and anti-Bax activities in the membrane.Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009 Aug;26(4):829-35. Sheng Wu Yi Xue Gong Cheng Xue Za Zhi. 2009. PMID: 19813621 Free PMC article.
-
Pore formation by dimeric Bak and Bax: an unusual pore?Philos Trans R Soc Lond B Biol Sci. 2017 Aug 5;372(1726):20160218. doi: 10.1098/rstb.2016.0218. Philos Trans R Soc Lond B Biol Sci. 2017. PMID: 28630157 Free PMC article. Review.
-
Mitochondria as the target of the pro-apoptotic protein Bax.Biochim Biophys Acta. 2006 Sep-Oct;1757(9-10):1301-11. doi: 10.1016/j.bbabio.2006.05.032. Epub 2006 May 27. Biochim Biophys Acta. 2006. PMID: 16836974 Review.
Cited by
-
Membrane-dependent amyloid aggregation of human BAX α9 (173-192).Protein Sci. 2021 May;30(5):1072-1080. doi: 10.1002/pro.4053. Epub 2021 Mar 12. Protein Sci. 2021. PMID: 33641228 Free PMC article.
-
Pyroglutamylated amyloid-β peptide reverses cross β-sheets by a prion-like mechanism.J Phys Chem B. 2014 May 29;118(21):5637-43. doi: 10.1021/jp412743s. Epub 2014 May 19. J Phys Chem B. 2014. PMID: 24802697 Free PMC article.
-
Structure of amyloid β25-35 in lipid environment and cholesterol-dependent membrane pore formation.Sci Rep. 2019 Feb 25;9(1):2689. doi: 10.1038/s41598-019-38749-7. Sci Rep. 2019. PMID: 30804528 Free PMC article.
-
The C-terminal Domains of Apoptotic BH3-only Proteins Mediate Their Insertion into Distinct Biological Membranes.J Biol Chem. 2016 Nov 25;291(48):25207-25216. doi: 10.1074/jbc.M116.733634. Epub 2016 Oct 7. J Biol Chem. 2016. PMID: 27758854 Free PMC article.
-
The CT20 peptide causes detachment and death of metastatic breast cancer cells by promoting mitochondrial aggregation and cytoskeletal disruption.Cell Death Dis. 2014 May 22;5(5):e1249. doi: 10.1038/cddis.2014.225. Cell Death Dis. 2014. PMID: 24853427 Free PMC article.
References
-
- Youle RJ, Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol. 2008;9:47–59. - PubMed
-
- Tait SW, Green DR. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol. 2010;11:621–632. - PubMed
-
- Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta. 2011;1813:521–531. - PubMed
-
- Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

