Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15
- PMID: 23104097
- PMCID: PMC3501574
- DOI: 10.1038/ni.2449
Mechanistic and structural insight into the functional dichotomy between IL-2 and IL-15
Abstract
Interleukin 15 (IL-15) and IL-2 have distinct immunological functions even though both signal through the receptor subunit IL-2Rβ and the common γ-chain (γ(c)). Here we found that in the structure of the IL-15-IL-15Rα-IL-2Rβ-γ(c) quaternary complex, IL-15 binds to IL-2Rβ and γ(c) in a heterodimer nearly indistinguishable from that of the IL-2-IL-2Rα-IL-2Rβ-γ(c) complex, despite their different receptor-binding chemistries. IL-15Rα substantially increased the affinity of IL-15 for IL-2Rβ, and this allostery was required for IL-15 trans signaling. Consistent with their identical IL-2Rβ-γ(c) dimer geometries, IL-2 and IL-15 showed similar signaling properties in lymphocytes, with any differences resulting from disparate receptor affinities. Thus, IL-15 and IL-2 induced similar signals, and the cytokine specificity of IL-2Rα versus IL-15Rα determined cellular responsiveness. Our results provide new insights for the development of specific immunotherapeutics based on IL-15 or IL-2.
Figures
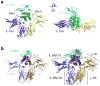

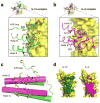
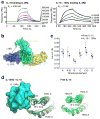
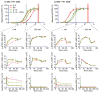
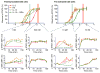
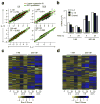
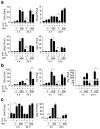
Comment in
-
IL-2 and IL-15 signaling complexes: different but the same.Nat Immunol. 2012 Dec;13(12):1141-2. doi: 10.1038/ni.2472. Nat Immunol. 2012. PMID: 23160210 No abstract available.
Similar articles
-
Crystal Structure of the interleukin-15.interleukin-15 receptor alpha complex: insights into trans and cis presentation.J Biol Chem. 2007 Dec 21;282(51):37191-204. doi: 10.1074/jbc.M706150200. Epub 2007 Oct 18. J Biol Chem. 2007. PMID: 17947230
-
Mechanistic and Structural Insights on the IL-15 System through Molecular Dynamics Simulations.Molecules. 2019 Sep 6;24(18):3261. doi: 10.3390/molecules24183261. Molecules. 2019. PMID: 31500206 Free PMC article.
-
Exploiting a natural conformational switch to engineer an interleukin-2 'superkine'.Nature. 2012 Mar 25;484(7395):529-33. doi: 10.1038/nature10975. Nature. 2012. PMID: 22446627 Free PMC article.
-
Increasing the biological activity of IL-2 and IL-15 through complexing with anti-IL-2 mAbs and IL-15Rα-Fc chimera.Immunol Lett. 2014 May-Jun;159(1-2):1-10. doi: 10.1016/j.imlet.2014.01.017. Epub 2014 Feb 7. Immunol Lett. 2014. PMID: 24512738 Review.
-
The soluble IL-2 receptor α/CD25 as a modulator of IL-2 function.Immunology. 2024 Mar;171(3):377-387. doi: 10.1111/imm.13723. Epub 2023 Nov 30. Immunology. 2024. PMID: 38037265 Review.
Cited by
-
Calibration of cell-intrinsic interleukin-2 response thresholds guides design of a regulatory T cell biased agonist.Elife. 2021 May 18;10:e65777. doi: 10.7554/eLife.65777. Elife. 2021. PMID: 34003116 Free PMC article.
-
Interleukin-2 activity can be fine tuned with engineered receptor signaling clamps.Immunity. 2015 May 19;42(5):826-38. doi: 10.1016/j.immuni.2015.04.018. Immunity. 2015. PMID: 25992859 Free PMC article.
-
Engineering a Single-Agent Cytokine/Antibody Fusion That Selectively Expands Regulatory T Cells for Autoimmune Disease Therapy.J Immunol. 2018 Oct 1;201(7):2094-2106. doi: 10.4049/jimmunol.1800578. Epub 2018 Aug 13. J Immunol. 2018. PMID: 30104245 Free PMC article.
-
Molecular and cellular factors determining the functional pleiotropy of cytokines.FEBS J. 2023 May;290(10):2525-2552. doi: 10.1111/febs.16420. Epub 2022 Mar 14. FEBS J. 2023. PMID: 35246947 Free PMC article. Review.
-
IL-15 Participates in the Pathogenesis of Polycystic Ovary Syndrome by Affecting the Activity of Granulosa Cells.Front Endocrinol (Lausanne). 2022 Feb 18;13:787876. doi: 10.3389/fendo.2022.787876. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35250857 Free PMC article.
References
-
- Waldmann TA. The biology of interleukin-2 and interleukin-15: implications for cancer therapy and vaccine design. Nat Rev Immunol. 2006;6:595–601. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

