Oxygen versus Reactive Oxygen in the Regulation of HIF-1α: The Balance Tips
- PMID: 23091723
- PMCID: PMC3474226
- DOI: 10.1155/2012/436981
Oxygen versus Reactive Oxygen in the Regulation of HIF-1α: The Balance Tips
Abstract
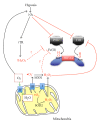
Hypoxia inducible factor (HIF) is known as the master regulator of the cellular response to hypoxia and is of pivotal importance during development as well as in human disease, particularly in cancer. It is composed of a constitutively expressed β subunit (HIF-1β) and an oxygen-regulated α subunit (HIF-1α and HIF-2α), whose stability is tightly controlled by a family of oxygen- and iron-dependent prolyl hydroxylase enzymes. Whether or not mitochondria-derived reactive oxygen species (ROS) are involved in the regulation of Hypoxia Inducible Factor-1α has been a matter of contention for the last 10 years, with equally compelling evidence in favor and against their contribution. A number of recent papers appear to tip the balance against a role for ROS. Thus, it has been demonstrated that HIF prolyl hydroxylases are unlikely to be physiological targets of ROS and that the increase in ROS that is associated with downregulation of Thioredoxin Reductase in hypoxia does not affect HIF-1α stabilization. Finally, the protein CHCHD4, which modulates cellular HIF-1α concentrations by promoting mitochondrial electron transport chain activity, has been proposed to exert its regulatory effect by affecting cellular oxygen availability. These reports are consistent with the hypothesis that mitochondria play a critical role in the regulation of HIF-1α by controlling intracellular oxygen concentrations.
Figures
Similar articles
-
Stabilization of hypoxia-inducible factor-1alpha protein in hypoxia occurs independently of mitochondrial reactive oxygen species production.J Biol Chem. 2010 Oct 8;285(41):31277-84. doi: 10.1074/jbc.M110.158485. Epub 2010 Jul 30. J Biol Chem. 2010. PMID: 20675386 Free PMC article.
-
NADPH oxidase-mitochondria axis-derived ROS mediate arsenite-induced HIF-1α stabilization by inhibiting prolyl hydroxylases activity.Toxicol Lett. 2014 Jan 13;224(2):165-74. doi: 10.1016/j.toxlet.2013.10.029. Epub 2013 Nov 1. Toxicol Lett. 2014. PMID: 24188932
-
Reactive oxygen species attenuate nitric-oxide-mediated hypoxia-inducible factor-1alpha stabilization.Free Radic Biol Med. 2006 Apr 15;40(8):1430-42. doi: 10.1016/j.freeradbiomed.2005.12.012. Epub 2006 Jan 6. Free Radic Biol Med. 2006. PMID: 16631533
-
The role of nitric oxide (NO) in stability regulation of hypoxia inducible factor-1alpha (HIF-1alpha).Curr Med Chem. 2003 May;10(10):845-55. doi: 10.2174/0929867033457746. Curr Med Chem. 2003. PMID: 12678687 Review.
-
HIF-prolyl hydroxylases and cardiovascular diseases.Toxicol Mech Methods. 2012 Jun;22(5):347-58. doi: 10.3109/15376516.2012.673088. Toxicol Mech Methods. 2012. PMID: 22424133 Review.
Cited by
-
Reactive Oxygen Species Mechanisms that Regulate Protein-Protein Interactions in Cancer.Int J Mol Sci. 2024 Aug 27;25(17):9255. doi: 10.3390/ijms25179255. Int J Mol Sci. 2024. PMID: 39273204 Free PMC article. Review.
-
Neutrophil-Derived Myeloperoxidase and Hypochlorous Acid Critically Contribute to 20-Hydroxyeicosatetraenoic Acid Increases that Drive Postischemic Angiogenesis.J Pharmacol Exp Ther. 2022 Jun;381(3):204-216. doi: 10.1124/jpet.121.001036. Epub 2022 Mar 19. J Pharmacol Exp Ther. 2022. PMID: 35306474 Free PMC article.
-
Molecular mechanisms of action and therapeutic uses of pharmacological inhibitors of HIF-prolyl 4-hydroxylases for treatment of ischemic diseases.Antioxid Redox Signal. 2014 Jun 1;20(16):2631-65. doi: 10.1089/ars.2013.5186. Epub 2013 Oct 31. Antioxid Redox Signal. 2014. PMID: 23992027 Free PMC article. Review.
-
HIF1α and metabolic reprogramming in inflammation.J Clin Invest. 2016 Oct 3;126(10):3699-3707. doi: 10.1172/JCI84431. Epub 2016 Aug 29. J Clin Invest. 2016. PMID: 27571407 Free PMC article. Review.
-
Protein trafficking in the mitochondrial intermembrane space: mechanisms and links to human disease.Biochem J. 2017 Jul 12;474(15):2533-2545. doi: 10.1042/BCJ20160627. Biochem J. 2017. PMID: 28701417 Free PMC article. Review.
References
-
- Chandel NS, McClintock DS, Feliciano CE, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1α during hypoxia: a mechanism of O2 sensing. The Journal of Biological Chemistry. 2000;275(33):25130–25138. - PubMed
-
- Jaakkola P, Mole DR, Tian YM, et al. Targeting of HIF-α to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292(5516):468–472. - PubMed
-
- Ivan M, Kondo K, Yang H, et al. HIFα targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292(5516):464–468. - PubMed
LinkOut - more resources
Full Text Sources

