Prophylactic effects of swimming exercise on autophagy-induced muscle atrophy in diabetic rats
- PMID: 23091517
- PMCID: PMC3469845
- DOI: 10.5625/lar.2012.28.3.171
Prophylactic effects of swimming exercise on autophagy-induced muscle atrophy in diabetic rats
Abstract
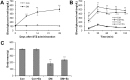
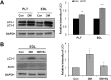
Diabetes decreases skeletal muscle mass and induces atrophy. However, the mechanisms by which hyperglycemia and insulin deficiency modify muscle mass are not well defined. In this study, we evaluated the effects of swimming exercise on muscle mass and intracellular protein degradation in diabetic rats, and proposed that autophagy inhibition induced by swimming exercise serves as a hypercatabolic mechanism in the skeletal muscles of diabetic rats, supporting a notion that swimming exercise could efficiently reverse the reduced skeletal muscle mass caused by diabetes. Adult male Sprague-Dawley rats were injected intraperitoneally with streptozotocin (60 mg/kg body weight) to induce diabetes and then submitted to 1 hr per day of forced swimming exercise, 5 days per week for 4 weeks. We conducted an intraperitoneal glucose tolerance test on the animals and measured body weight, skeletal muscle mass, and protein degradation and examined the level of autophagy in the isolated extensor digitorum longus, plantaris, and soleus muscles. Body weight and muscle tissue mass were higher in the exercising diabetic rats than in control diabetic rats that remained sedentary. Compared to control rats, exercising diabetic rats had lower blood glucose levels, increased intracellular contractile protein expression, and decreased autophagic protein expression. We conclude that swimming exercise improves muscle mass in diabetes-induced skeletal muscle atrophy, suggesting the activation of autophagy in diabetes contributes to muscle atrophy through hypercatabolic metabolism and that aerobic exercise, by suppressing autophagy, may modify or reverse skeletal muscle wasting in diabetic patients.
Keywords: Autophagy; diabetes; muscle atrophy; prophylactic effect; swimming exercise.
Figures

Similar articles
-
[The effects of aerobic exercise on ERK1/2 activity in skeletal muscle of type 2 diabetic rats].Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2017 Jan 8;33(1):33-37. doi: 10.12047/j.cjap.5448.2017.008. Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2017. PMID: 29926604 Chinese.
-
Effects of swimming training at the intensity equivalent to aerobic/anaerobic metabolic transition in alloxan diabetic rats.J Diabetes Complications. 2007 Jul-Aug;21(4):258-64. doi: 10.1016/j.jdiacomp.2006.07.007. J Diabetes Complications. 2007. PMID: 17616357
-
Aerobic Exercise Ameliorates Muscle Atrophy Induced by Methylglyoxal via Increasing Gastrocnemius and Extensor Digitorum Longus Muscle Sensitivity.Biomol Ther (Seoul). 2023 Sep 1;31(5):573-582. doi: 10.4062/biomolther.2023.130. Epub 2023 Aug 11. Biomol Ther (Seoul). 2023. PMID: 37562979 Free PMC article.
-
Effects of high-intensity interval training (HIIT) on skeletal muscle atrophy, function, and myokine profile in diabetic myopathy.Cytokine. 2023 Sep;169:156279. doi: 10.1016/j.cyto.2023.156279. Epub 2023 Jun 15. Cytokine. 2023. PMID: 37329818
-
Differential control of muscle mass in type 1 and type 2 diabetes mellitus.Cell Mol Life Sci. 2015 Oct;72(20):3803-17. doi: 10.1007/s00018-015-1954-7. Epub 2015 Jun 20. Cell Mol Life Sci. 2015. PMID: 26091746 Free PMC article. Review.
Cited by
-
Autophagic cellular responses to physical exercise in skeletal muscle.Sports Med. 2014 May;44(5):625-40. doi: 10.1007/s40279-013-0140-z. Sports Med. 2014. PMID: 24549475 Review.
-
Effects of exercise on cellular and tissue aging.Aging (Albany NY). 2021 May 13;13(10):14522-14543. doi: 10.18632/aging.203051. Epub 2021 May 13. Aging (Albany NY). 2021. PMID: 34001677 Free PMC article. Review.
-
Heat shock response and autophagy--cooperation and control.Autophagy. 2015;11(2):200-13. doi: 10.1080/15548627.2015.1009776. Autophagy. 2015. PMID: 25714619 Free PMC article. Review.
-
The high-intensity interval training (HIIT) and curcumin supplementation can positively regulate the autophagy pathway in myocardial cells of STZ-induced diabetic rats.BMC Res Notes. 2023 Feb 25;16(1):21. doi: 10.1186/s13104-023-06295-1. BMC Res Notes. 2023. PMID: 36841820 Free PMC article.
-
Association of sericin and swimming on the phenotype, motor plate, and functionality of the denervated plantar muscle of Wistar rats.J Exerc Rehabil. 2018 Feb 26;14(1):24-31. doi: 10.12965/jer.1835138.569. eCollection 2018 Feb. J Exerc Rehabil. 2018. PMID: 29511649 Free PMC article.
References
-
- Sun Z, Liu L, Liu N, Liu Y. Muscular response and adaptation to diabetes mellitus. Front Biosci. 2008;13:4765–4794. - PubMed
-
- Lecker SH, Solomon V, Mitch WE, Goldberg AL. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J Nutr. 1999;129(1S Suppl):227S–237S. - PubMed
-
- Emery PW. Cachexia in experimental models. Nutrition. 1999;15(7-8):600–603. - PubMed
-
- Costelli P, Baccino FM. Cancer cachexia: from experimental models to patient management. Curr Opin Clin Nutr Metab Care. 2000;3(3):177–181. - PubMed
-
- Attaix D, Combaret L, Tilignac T, Taillandier D. Adaptation of the ubiquitin-proteasome proteolytic pathway in cancer cachexia. Mol Biol Rep. 1999;26(1-2):77–82. - PubMed

