Localized mucosal response to intranasal live attenuated influenza vaccine in adults
- PMID: 23087433
- PMCID: PMC3571238
- DOI: 10.1093/infdis/jis641
Localized mucosal response to intranasal live attenuated influenza vaccine in adults
Abstract
Background: Influenza virus infection is a major public health burden worldwide. Available vaccines include the inactivated intramuscular trivalent vaccine and, more recently, an intranasal live attenuated influenza vaccine (LAIV). The measure of successful vaccination with the inactivated vaccine is a systemic rise in immunoglobulin G (IgG) level, but for the LAIV no such correlate has been established.
Methods: Seventy-nine subjects were given the LAIV FluMist. Blood was collected prior to vaccination and 3 days and 30 days after vaccination. Nasal wash was collected 3 days and 30 days after vaccination. Responses were measured systemically and in mucosal secretions for cytokines, cell activation profiles, and antibody responses.
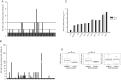
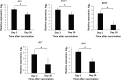
Results: Only 9% of subjects who received LAIV seroconverted, while 33% of patients developed at least a 2-fold increase in influenza virus-specific immunoglobulin A (IgA) antibodies in nasal wash. LAIV induced a localized inflammation, as suggested by increased expression of interferon-response genes in mucosal RNA and increased granulocyte colony-stimulating factor (G-CSF) and IP-10 in nasal wash. Interestingly, patients who seroconverted had significantly lower serum levels of G-CSF before vaccination.
Conclusions: Protection by LAIV is likely provided through mucosal IgA and not by increases in systemic IgG. LAIV induces local inflammation. Seroconversion is achieved in a small fraction of subjects with a lower serum G-CSF level.
Figures


Similar articles
-
Pre-existing influenza-specific nasal IgA or nasal viral infection does not affect live attenuated influenza vaccine immunogenicity in children.Clin Exp Immunol. 2021 Apr;204(1):125-133. doi: 10.1111/cei.13564. Epub 2021 Jan 13. Clin Exp Immunol. 2021. PMID: 33314126 Free PMC article. Clinical Trial.
-
Oral Fluids as a Live-Animal Sample Source for Evaluating Cross-Reactivity and Cross-Protection following Intranasal Influenza A Virus Vaccination in Pigs.Clin Vaccine Immunol. 2015 Oct;22(10):1109-20. doi: 10.1128/CVI.00358-15. Epub 2015 Aug 19. Clin Vaccine Immunol. 2015. PMID: 26291090 Free PMC article.
-
Rationale for vaccination with trivalent or quadrivalent live attenuated influenza vaccines: Protective vaccine efficacy in the ferret model.PLoS One. 2018 Dec 3;13(12):e0208028. doi: 10.1371/journal.pone.0208028. eCollection 2018. PLoS One. 2018. PMID: 30507951 Free PMC article.
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
-
Live attenuated intranasal influenza vaccine.Hum Vaccin Immunother. 2012 Jan;8(1):76-80. doi: 10.4161/hv.8.1.18809. Epub 2012 Jan 1. Hum Vaccin Immunother. 2012. PMID: 22251995 Review.
Cited by
-
Immune Responses Elicited by Live Attenuated Influenza Vaccines as Correlates of Universal Protection against Influenza Viruses.Vaccines (Basel). 2021 Apr 7;9(4):353. doi: 10.3390/vaccines9040353. Vaccines (Basel). 2021. PMID: 33916924 Free PMC article. Review.
-
Differences in antibody responses between trivalent inactivated influenza vaccine and live attenuated influenza vaccine correlate with the kinetics and magnitude of interferon signaling in children.J Infect Dis. 2014 Jul 15;210(2):224-33. doi: 10.1093/infdis/jiu079. Epub 2014 Feb 4. J Infect Dis. 2014. PMID: 24495909 Free PMC article.
-
Immunogenicity of chimeric haemagglutinin-based, universal influenza virus vaccine candidates: interim results of a randomised, placebo-controlled, phase 1 clinical trial.Lancet Infect Dis. 2020 Jan;20(1):80-91. doi: 10.1016/S1473-3099(19)30393-7. Epub 2019 Oct 17. Lancet Infect Dis. 2020. PMID: 31630990 Free PMC article. Clinical Trial.
-
Comparative Immunogenicity of the 2014-2015 Northern Hemisphere Trivalent IIV and LAIV against Influenza A Viruses in Children.Vaccines (Basel). 2019 Aug 12;7(3):87. doi: 10.3390/vaccines7030087. Vaccines (Basel). 2019. PMID: 31408963 Free PMC article.
-
Mucosal CD8+ T cell responses induced by an MCMV based vaccine vector confer protection against influenza challenge.PLoS Pathog. 2019 Sep 16;15(9):e1008036. doi: 10.1371/journal.ppat.1008036. eCollection 2019 Sep. PLoS Pathog. 2019. PMID: 31525249 Free PMC article.
References
-
- Molinari NA, Ortega-Sanchez IR, Messonnier ML, et al. The annual impact of seasonal influenza in the US: measuring disease burden and costs. Vaccine. 2007;25:5086–96. - PubMed
-
- Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–62. - PubMed
-
- Belshe R, Lee MS, Walker RE, Stoddard J, Mendelman PM. Safety, immunogenicity and efficacy of intranasal, live attenuated influenza vaccine. Expert Rev Vaccines. 2004;3:643–54. - PubMed
-
- Esposito S, Montinaro V, Groppali E, Tenconi R, Semino M, Principi N. Live attenuated intranasal influenza vaccine. Hum Vaccin Immunother. 2012;8:1–5. - PubMed
-
- Carter NJ, Curran MP. Live attenuated influenza vaccine (FluMist(R); Fluenz): a review of its use in the prevention of seasonal influenza in children and adults. Drugs. 2011;71:1591–622. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

