Development of embryonic stem cells in recombinant kidneys
- PMID: 23086378
- PMCID: PMC3562253
- DOI: 10.4161/org.22597
Development of embryonic stem cells in recombinant kidneys
Abstract
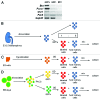
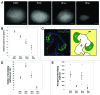
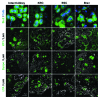
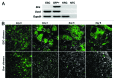
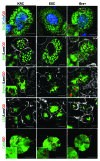
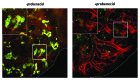
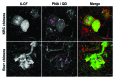
Embryonic stem cells (ESC) are self-renewing and can generate all cell types during normal development. Previous studies have begun to explore fates of ESCs and their mesodermal derivatives after injection into explanted intact metanephric kidneys and neonatal kidneys maturing in vivo. Here, we exploited a recently described recombinant organ culture model, mixing fluorescent quantum dot labeled mouse exogenous cells with host metanephric cells. We compared abilities of undifferentiated ESCs with ESC-derived mesodermal or non-mesodermal cells to contribute to tissue compartments within recombinant, chimeric metanephroi. ESC-derived mesodermal cells downregulated Oct4, a marker of undifferentiated cells, and, as assessed by locations of quantum dots, contributed to Wilms' tumor 1-expressing forming nephrons, synaptopodin-expressing glomeruli, and organic ion-transporting tubular epithelia. Similar results were observed when labeled native metanephric cells were recombined with host cells. In striking contrast, non-mesodermal ESC-derived cells strongly inhibited growth of embryonic kidneys, while undifferentiated ESCs predominantly formed Oct4 expressing colonies between forming nephrons and glomeruli. These findings clarify the conclusion that ESC-derived mesodermal cells have functional nephrogenic potential, supporting the idea that they could potentially replace damaged epithelia in diseased kidneys. On the other hand, undifferentiated ESCs and non-mesodermal precursors derived from ESCs would appear to be less suitable materials for use in kidney cell therapies.
Keywords: differentiation; embryonic stem cell; kidney; mesoderm; metanephros; nephrogenesis; organic anion transport; quantum dot; rudiment.
Figures

Similar articles
-
Mouse embryonic stem cell-derived embryoid bodies generate progenitors that integrate long term into renal proximal tubules in vivo.J Am Soc Nephrol. 2007 Jun;18(6):1709-20. doi: 10.1681/ASN.2006101078. Epub 2007 May 2. J Am Soc Nephrol. 2007. PMID: 17475814
-
Locust bean gum as an alternative polymeric coating for embryonic stem cell culture.Mater Sci Eng C Mater Biol Appl. 2014 Jul 1;40:336-44. doi: 10.1016/j.msec.2014.04.022. Epub 2014 Apr 16. Mater Sci Eng C Mater Biol Appl. 2014. PMID: 24857501
-
Quantitative Oct4 overproduction in mouse embryonic stem cells results in prolonged mesoderm commitment during hematopoietic differentiation in vitro.Stem Cells. 2006 Aug;24(8):1937-45. doi: 10.1634/stemcells.2005-0067. Epub 2006 May 11. Stem Cells. 2006. PMID: 16690781
-
Stem cells in the embryonic kidney.Kidney Int. 2008 Apr;73(8):913-7. doi: 10.1038/sj.ki.5002784. Epub 2008 Jan 16. Kidney Int. 2008. PMID: 18200005 Review.
-
Generation of chimeric kidneys using progenitor cell replacement: Oshima Award Address 2021.Clin Exp Nephrol. 2022 Jun;26(6):491-500. doi: 10.1007/s10157-022-02191-3. Epub 2022 Feb 9. Clin Exp Nephrol. 2022. PMID: 35138500 Free PMC article. Review.
Cited by
-
Embryonic Stem Cells Derived Kidney Organoids as Faithful Models to Target Programmed Nephrogenesis.Sci Rep. 2018 Nov 9;8(1):16618. doi: 10.1038/s41598-018-34995-3. Sci Rep. 2018. PMID: 30413738 Free PMC article.
-
Functional comparison of distinct Brachyury+ states in a renal differentiation assay.Biol Open. 2018 May 17;7(5):bio031799. doi: 10.1242/bio.031799. Biol Open. 2018. PMID: 29666052 Free PMC article.
-
Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors.J Am Soc Nephrol. 2013 Sep;24(9):1424-34. doi: 10.1681/ASN.2012121143. Epub 2013 Jun 13. J Am Soc Nephrol. 2013. PMID: 23766537 Free PMC article.
-
Transport of organic anions and cations in murine embryonic kidney development and in serially-reaggregated engineered kidneys.Sci Rep. 2015 Mar 13;5:9092. doi: 10.1038/srep09092. Sci Rep. 2015. PMID: 25766625 Free PMC article.
-
Autologous Cells for Kidney Bioengineering.Curr Transplant Rep. 2016;3:207-220. doi: 10.1007/s40472-016-0107-8. Epub 2016 Jun 9. Curr Transplant Rep. 2016. PMID: 27547698 Free PMC article. Review.
References
-
- Robertson E, ed. Embryo-derived stem cell lines. In teratocarcinomas and embryonic stem cells; a protocal approach. IRL Press Limited; Oxford, 1987.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
