Correlation between circulating myeloid-derived suppressor cells and Th17 cells in esophageal cancer
- PMID: 23082063
- PMCID: PMC3471115
- DOI: 10.3748/wjg.v18.i38.5454
Correlation between circulating myeloid-derived suppressor cells and Th17 cells in esophageal cancer
Abstract
Aim: To perform a comprehensive investigation into the potential correlation between circulating myeloid-derived suppressor cells (MDSCs) and Th17 cells in esophageal cancer (ECA).
Methods: A total of 31 patients newly diagnosed with ECA and 26 healthy subjects were included in the current study. The frequencies of MDSCs and Th17 cells in peripheral blood were determined by flow cytometry. The mRNA expression of cytokines, arginase 1 (Arg1) and inducible NO synthase (iNOS) in peripheral blood mononuclear cells (PBMCs) and plasma Arg1 were assessed by real-time polymerase chain reaction and enzyme-linked immunosorbent assay, respectively.
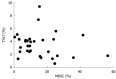
Results: There was an increased prevalence of MDSCs in the peripheral blood from ECA patients (15.21% ± 2.25%) when compared with healthy control (HC) (1.10% ± 0.12%, P < 0.0001). The plasma levels of Arg1 in ECA patients were significantly higher than those in HC (28.28 ± 4.10 ng/mL vs 9.57 ± 1.51 ng/mL, P = 0.0003). iNOS mRNA levels in the peripheral blood of ECA patients also showed a threefold increase compared with HC (P = 0.0162). The frequencies of Th17 cells (CD4⁺IL-17A⁺) were significantly elevated in ECA patients versus HC (3.50% ± 0.33% vs 1.82% ± 0.19%, P = 0.0001). Increased mRNA expression of IL-17 and ROR-γt was also observed in ECA patients compared with HC (P = 0.0041 and P = 0.0004, respectively), while the mRNA expression of IL-6 and tumor necrosis factor-α (TNF-α) showed significant decreases (P = 0.0049 and P < 0.0001, respectively). No obvious correlations were found between the frequencies of MDSCs and Th17 cells in the peripheral blood from ECA patients(r = -0.1725, P = 0.3534). Arg1 mRNA levels were positively correlated with levels of IL-6 (r = 0.6404, P = 0.0031) and TNF-α (r = 0.7646, P = 0.0001). Similarly, iNOS mRNA levels were also positively correlated with levels of IL-6 (r = 0.6782, P = 0.0007) and TNF-α (r = 0.7633, P < 0.0001).
Conclusion: This study reveals the relationship between circulating MDSCs and Th17 cells, which may lead to new immunotherapy approaches for ECA based on the associated metabolites and cytokines.
Keywords: Arginase I; Esophageal cancer; Inducible NO synthase; Myeloid-derived suppressor cells; Peripheral blood mononuclear cells; Th17 cells.
Figures



Similar articles
-
Increased circulating myeloid-derived suppressor cells correlated negatively with Th17 cells in patients with rheumatoid arthritis.Scand J Rheumatol. 2013;42(2):85-90. doi: 10.3109/03009742.2012.716450. Epub 2012 Nov 6. Scand J Rheumatol. 2013. PMID: 23126644
-
Elevated Th17 cells accompanied by decreased regulatory T cells and cytokine environment in infants with biliary atresia.Pediatr Surg Int. 2013 Dec;29(12):1249-60. doi: 10.1007/s00383-013-3421-6. Pediatr Surg Int. 2013. PMID: 24122073
-
Infiltration of alternatively activated macrophages in cancer tissue is associated with MDSC and Th2 polarization in patients with esophageal cancer.PLoS One. 2014 Aug 21;9(8):e104453. doi: 10.1371/journal.pone.0104453. eCollection 2014. PLoS One. 2014. PMID: 25144454 Free PMC article.
-
Circulating myeloid-derived suppressor cells in patients with pancreatic cancer.Hepatobiliary Pancreat Dis Int. 2016 Feb;15(1):99-105. doi: 10.1016/s1499-3872(15)60413-1. Hepatobiliary Pancreat Dis Int. 2016. PMID: 26818550
-
Interplay between myeloid-derived suppressor cells (MDSCs) and Th17 cells: foe or friend?Oncotarget. 2016 Jun 7;7(23):35490-6. doi: 10.18632/oncotarget.8204. Oncotarget. 2016. PMID: 27007054 Free PMC article. Review.
Cited by
-
Mechanisms of tumor immunosuppressive microenvironment formation in esophageal cancer.World J Gastroenterol. 2024 Apr 28;30(16):2195-2208. doi: 10.3748/wjg.v30.i16.2195. World J Gastroenterol. 2024. PMID: 38690024 Free PMC article. Review.
-
Vaccination with dendritic cells loaded with allogeneic brain tumor cells for recurrent malignant brain tumors induces a CD4(+)IL17(+) response.J Immunother Cancer. 2014 Feb 18;2:4. doi: 10.1186/2051-1426-2-4. eCollection 2014. J Immunother Cancer. 2014. PMID: 24829761 Free PMC article.
-
Immune determinants of Barrett's progression to esophageal adenocarcinoma.JCI Insight. 2021 Jan 11;6(1):e143888. doi: 10.1172/jci.insight.143888. JCI Insight. 2021. PMID: 33290281 Free PMC article.
-
Impact of the Tumor Microenvironment for Esophageal Tumor Development-An Opportunity for Prevention?Cancers (Basel). 2022 Apr 30;14(9):2246. doi: 10.3390/cancers14092246. Cancers (Basel). 2022. PMID: 35565378 Free PMC article. Review.
-
Increased level of myeloid-derived suppressor cells, programmed death receptor ligand 1/programmed death receptor 1, and soluble CD25 in Sokal high risk chronic myeloid leukemia.PLoS One. 2013;8(1):e55818. doi: 10.1371/journal.pone.0055818. Epub 2013 Jan 31. PLoS One. 2013. PMID: 23383287 Free PMC article.
References
-
- Brandau S, Trellakis S, Bruderek K, Schmaltz D, Steller G, Elian M, Suttmann H, Schenck M, Welling J, Zabel P, et al. Myeloid-derived suppressor cells in the peripheral blood of cancer patients contain a subset of immature neutrophils with impaired migratory properties. J Leukoc Biol. 2011;89:311–317. - PubMed
-
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. - PubMed
-
- Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S, Zanon A, Rossi CR, Nitti D, Bronte V, et al. IL4Ralpha+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol. 2009;182:6562–6568. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

