Cytokines of the γ(c) family control CD4+ T cell differentiation and function
- PMID: 23080204
- PMCID: PMC4825860
- DOI: 10.1038/ni.2431
Cytokines of the γ(c) family control CD4+ T cell differentiation and function
Abstract
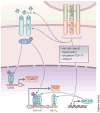
Naive CD4(+) T cells undergo massive proliferation and differentiation into at least four distinct helper T cell subsets after recognition of foreign antigen-derived peptides presented by dendritic cells. Each helper T cell subset expresses a distinct set of genes that encode unique transcription factor(s), as well as hallmark cytokines. The cytokine environment created by activated CD4(+) T cells, dendritic cells and/or other cell types during the course of differentiation is a major determinant for the helper T cell fate. This Review focuses on the role of cytokines of the common γ-chain (γ(c)) family in the determination of the effector helper T cell phenotype that naive CD4(+) T cells adopt after being activated and in the function of these helper T cells.
Figures
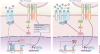
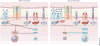
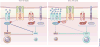
Similar articles
-
Early signaling events that underlie fate decisions of naive CD4(+) T cells toward distinct T-helper cell subsets.Immunol Rev. 2013 Mar;252(1):12-23. doi: 10.1111/imr.12032. Immunol Rev. 2013. PMID: 23405892 Free PMC article. Review.
-
The role of Notch in the differentiation of CD4⁺ T helper cells.Curr Top Microbiol Immunol. 2012;360:115-34. doi: 10.1007/82_2012_227. Curr Top Microbiol Immunol. 2012. PMID: 22653552 Review.
-
Human T helper cell differentiation is regulated by the combined action of cytokines and accessory cell-dependent costimulatory signals.J Immunol. 1997 Mar 15;158(6):2654-62. J Immunol. 1997. PMID: 9058798
-
Regulation of human helper T cell subset differentiation by cytokines.Curr Opin Immunol. 2015 Jun;34:130-6. doi: 10.1016/j.coi.2015.03.007. Epub 2015 Apr 11. Curr Opin Immunol. 2015. PMID: 25879814 Free PMC article. Review.
-
Mouse Naïve CD4+ T Cell Isolation and In vitro Differentiation into T Cell Subsets.J Vis Exp. 2015 Apr 16;(98):52739. doi: 10.3791/52739. J Vis Exp. 2015. PMID: 25938923 Free PMC article.
Cited by
-
T helper 2 (Th2) cell differentiation, type 2 innate lymphoid cell (ILC2) development and regulation of interleukin-4 (IL-4) and IL-13 production.Cytokine. 2015 Sep;75(1):14-24. doi: 10.1016/j.cyto.2015.05.010. Epub 2015 Jun 1. Cytokine. 2015. PMID: 26044597 Free PMC article. Review.
-
Common γ-chain blocking peptide reduces in vitro immune activation markers in HTLV-1-associated myelopathy/tropical spastic paraparesis.Proc Natl Acad Sci U S A. 2015 Sep 1;112(35):11030-5. doi: 10.1073/pnas.1412626112. Epub 2015 Aug 17. Proc Natl Acad Sci U S A. 2015. PMID: 26283355 Free PMC article.
-
mTOR regulates neuroprotective effect of immunized CD4+Foxp3+ T cells in optic nerve ischemia.Sci Rep. 2016 Nov 25;6:37805. doi: 10.1038/srep37805. Sci Rep. 2016. PMID: 27886260 Free PMC article.
-
B7-H3 Promotes Pathogenesis of Autoimmune Disease and Inflammation by Regulating the Activity of Different T Cell Subsets.PLoS One. 2015 Jun 11;10(6):e0130126. doi: 10.1371/journal.pone.0130126. eCollection 2015. PLoS One. 2015. PMID: 26065426 Free PMC article.
-
Common gamma chain cytokines in combinatorial immune strategies against cancer.Immunol Lett. 2016 Jan;169:61-72. doi: 10.1016/j.imlet.2015.11.007. Epub 2015 Nov 17. Immunol Lett. 2016. PMID: 26597610 Free PMC article. Review.
References
-
- Dubois S, Mariner J, Waldmann TA, Tagaya Y. IL-15Rα recycles and presents IL-15 in trans to neighboring cells. Immunity. 2002;17:537–547. - PubMed
-
- Rautajoki KJ, Kylaniemi MK, Raghav SK, Rao K, Lahesmaa R. An insight into molecular mechanisms of human T helper cell differentiation. Ann Med. 2008;40:322–335. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

