Chemotherapy for metastatic and recurrent cervical cancer
- PMID: 23076924
- PMCID: PMC6457617
- DOI: 10.1002/14651858.CD006469.pub2
Chemotherapy for metastatic and recurrent cervical cancer
Abstract
Background: Cervical cancer is the second most common cancer among women up to 65 years of age and is the most frequent cause of death from gynaecological cancers worldwide. A woman's risk of developing cervical cancer by 65 years of age ranges from 0.69% in developed countries to 1.38% in developing countries. Although screening by Pap smear should mean early detection at a curable stage for most women, many still present with advanced or metastatic disease with a worse prognosis. The addition of platinum-based chemotherapy to radiotherapy has improved outcome compared to radiotherapy alone; however, 30% to 50% fail to respond to treatment or develop recurrent disease. There are no standard treatment options for these patients, although platinum-based chemotherapy is frequently used and trials are on-going.
Objectives: To compare different types and combinations of cytotoxic chemotherapy for the treatment of metastatic/recurrent cervical cancer.
Search methods: We searched the Cochrane Gynaecological Cancer Group Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL, Issue 1, 2012), MEDLINE (1950 to January 2012) and EMBASE (1980 to January 2012). The reference lists from these and those of review articles were also checked.
Selection criteria: All randomised controlled trials (RCTs) involving chemotherapy for metastatic/recurrent cervical cancer. Trials involving radiotherapy, chemoradiotherapy, intra-arterial chemotherapy, biological agents or immunomodulators were excluded.
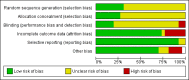
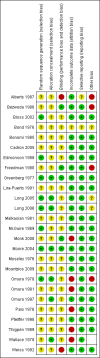
Data collection and analysis: Three review authors independently reviewed trials for inclusion and data extraction and assessed risk of bias.
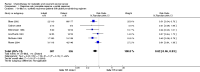
Main results: There were no data comparing best supportive care with chemotherapy. Cisplatin-based regimens are the most widely used and therefore we have concentrated on these trials. In terms of response rates some non-platinum regimens are equivalent but toxicity is higher. The most common cisplatin regimen was 50 mg/m(2) day 1 q21days. Higher doses had similar survivals. There was no direct comparison between single-agent cisplatin and carboplatin. Overall survival (OS) and progression-free survival (PFS) were not adequately reported and quality of life (QoL) outcomes were incompletely documented. Combination regimens were more toxic than single agents, but in the limited reported data this did not appear to adversely affect QoL.No significant difference in response rate by site of recurrence was found, although there was a trend towards improved response when the main site of disease was beyond the previously irradiated pelvis.
Authors' conclusions: Combination cisplatin-based chemotherapy could be a viable option for patients of good performance status with recurrent/metastatic cervical cancer, but further trials that report adequate survival and QoL data are sought. Response rates and improvements in survival are low. Cisplatin-based combinations have significant toxicity. Outcomes are poor and novel cytotoxic/biological agents and optimal scheduling need further investigation. Future trials need to stratify for and perform planned subgroup analysis with respect to previous treatment and site of recurrence.
Conflict of interest statement
No declarations of interest to declare.
Figures











Update of
- doi: 10.1002/14651858.CD006469
Similar articles
-
Adjuvant platinum-based chemotherapy for early stage cervical cancer.Cochrane Database Syst Rev. 2016 Nov 22;11(11):CD005342. doi: 10.1002/14651858.CD005342.pub4. Cochrane Database Syst Rev. 2016. PMID: 27873308 Free PMC article. Review.
-
Adjuvant platinum-based chemotherapy for early stage cervical cancer.Cochrane Database Syst Rev. 2012 Jun 13;6(6):CD005342. doi: 10.1002/14651858.CD005342.pub3. Cochrane Database Syst Rev. 2012. Update in: Cochrane Database Syst Rev. 2016 Nov 22;11:CD005342. doi: 10.1002/14651858.CD005342.pub4. PMID: 22696349 Free PMC article. Updated. Review.
-
Chemotherapy for advanced gastric cancer.Cochrane Database Syst Rev. 2017 Aug 29;8(8):CD004064. doi: 10.1002/14651858.CD004064.pub4. Cochrane Database Syst Rev. 2017. PMID: 28850174 Free PMC article. Review.
-
Hysterectomy with radiotherapy or chemotherapy or both for women with locally advanced cervical cancer.Cochrane Database Syst Rev. 2015 Apr 7;(4):CD010260. doi: 10.1002/14651858.CD010260.pub2. Cochrane Database Syst Rev. 2015. Update in: Cochrane Database Syst Rev. 2022 Aug 22;8:CD010260. doi: 10.1002/14651858.CD010260.pub3. PMID: 25847525 Updated. Review.
-
Cisplatin versus carboplatin in combination with third-generation drugs for advanced non-small cell lung cancer.Cochrane Database Syst Rev. 2020 Jan 13;1(1):CD009256. doi: 10.1002/14651858.CD009256.pub3. Cochrane Database Syst Rev. 2020. PMID: 31930743 Free PMC article.
Cited by
-
Synergistic effects of sodium butyrate and cisplatin against cervical carcinoma in vitro and in vivo.Front Oncol. 2022 Oct 21;12:999667. doi: 10.3389/fonc.2022.999667. eCollection 2022. Front Oncol. 2022. PMID: 36338704 Free PMC article.
-
Primary debulking surgery for metastatic cervical adenocarcinoma: A case report.Gynecol Oncol Rep. 2015 Oct 27;14:23-5. doi: 10.1016/j.gore.2015.09.004. eCollection 2015 Nov. Gynecol Oncol Rep. 2015. PMID: 26793767 Free PMC article.
-
Is Cytoreductive Surgery Possible in Cervical Cancer Peritoneal Carcinomatosis?Clin Med Insights Oncol. 2021 Dec 16;15:11795549211065308. doi: 10.1177/11795549211065308. eCollection 2021. Clin Med Insights Oncol. 2021. PMID: 34949947 Free PMC article.
-
Response to chemotherapy in patients with recurrent rectal cancer in previously irradiated area.Int J Colorectal Dis. 2015 Aug;30(8):1075-80. doi: 10.1007/s00384-015-2270-2. Epub 2015 Jun 17. Int J Colorectal Dis. 2015. PMID: 26077667 Free PMC article.
-
Primary lung adenocarcinoma harboring upper mediastinal lymphatic skip metastasis of cervical squamous cell carcinoma: A case report and literature review.Oncol Lett. 2024 Aug 7;28(4):481. doi: 10.3892/ol.2024.14614. eCollection 2024 Oct. Oncol Lett. 2024. PMID: 39161330 Free PMC article.
References
References to studies included in this review
Alberts 1987 {published data only}
-
- Alberts DS, Kronmal R, Baker LH, Stock‐Novack DL, Surwit EA, Boutselis JG, et al. Phase II randomized trial of cisplatin chemotherapy regimens in the treatment of recurrent or metastatic squamous cell cancer of the cervix: a Southwest Oncology Group Study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 1987;5(11):1791‐5. [PUBMED: 2445932] - PubMed
Bezwoda 1986 {published data only}
-
- Bezwoda WR, Nissenbaum M, Derman DP. Treatment of metastatic and recurrent cervix cancer with chemotherapy: a randomised trial comparing hydroxyurea with cisdiamminedichloro‐platinum plus methotrexate. Medical and Pediatric Oncology 1986;14(1):17‐9. [PUBMED: 3512970] - PubMed
Bloss 2002 {published data only}
-
- Bloss JD, Blessing JA, Behrens BC, Mannel RS, Rader JS, Sood AK, et al. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 2002;20(7):1832‐7. [PUBMED: 11919241] - PubMed
Bond 1976 {published data only}
-
- Bond WH, Arthur K, Banks AJ, Freeman WE, Holme GM, Newsholme GA, et al. Combination chemotherapy in the treatment of advanced squamous cell carcinoma of the cervix. Clinical Oncology 1976;2(2):173‐8. [PUBMED: 60188] - PubMed
Bonomi 1985 {published data only}
-
- Bonomi P, Blessing JA, Stehman FB, DiSaia PJ, Walton L, Major FJ. Randomized trial of three cisplatin dose schedules in squamous‐cell carcinoma of the cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 1985;3(8):1079‐85. [PUBMED: 3894589] - PubMed
Cadron 2005 {published data only}
-
- Cadron I, Jakobsen A, Vergote I. Report of an early stopped randomized trial comparing cisplatin vs. cisplatin/ifosfamide/ 5‐fluorouracil in recurrent cervical cancer. Gynecologic and Obstetric Investigation 2005;59(3):126‐9. [PUBMED: 15604591] - PubMed
Edmonson 1988 {published data only}
-
- Edmonson JH, Johnson PS, Wieand HS, Malkasian GD, Cullinan SA, Brown LD, et al. Phase II studies of bleomycin, cyclophosphamide, doxorubicin and cisplatin, and bleomycin and cisplatin in advanced cervical carcinoma. American Journal of Clinical Oncology 1988;11(2):149‐51. [PUBMED: 2451883] - PubMed
Freedman 1980 {published data only}
-
- Freedman RS, Herson J, Wharton JT, Rutledge FN. Single‐agent chemotherapy for recurrent carcinoma of the cervix. Cancer Clinical Trials 1980;3(4):345‐50. [PUBMED: 6775827] - PubMed
Greenberg 1977 {published data only}
-
- Greenberg BR, Kardinal CG, Pajak TF, Bateman JR. Adriamycin versus adriamycin and bleomycin in advanced epidermoid carcinoma of the cervix. Cancer Treatment Reports 1977;61(7):1383‐4. [PUBMED: 73417] - PubMed
Lira‐Puerto 1991 {published data only}
-
- Lira‐Puerto V, Silva A, Morris M, Martinez R, Groshen S, Morales‐Canfield F, et al. Phase II trial of carboplatin or iproplatin in cervical cancer. Cancer Chemotherapy and Pharmacology 1991;28(5):391‐6. [PUBMED: 1914084] - PubMed
Long 2005 {published data only}
-
- Brave M, Dagher R, Farrel A, Ramchandani R, Gobburu J, Booth R, et al. Topotecan in combination with cisplatin for the treatment of stage IVB, recurrent or persistent cervical cancer. Oncology 2006;20:1401‐16. - PubMed
-
- Long HJ 3rd, Bundy BN, Grendys EC Jr, Benda JA, McMeekin DS, Sorosky J, et al. Randomized phase III trial of cisplatin with or without topotecan in carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 2005;23(21):4626‐33. [PUBMED: 15911865] - PubMed
-
- Monk BJ, Huang HQ, Cella D, Long HJ 3rd. Quality of life outcomes from a randomised phase III trial of cisplatin with or without topotecan in advanced carcinoma of the cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology 2005;23(21):4617‐25. - PubMed
Long 2006 {published data only}
-
- Long HJ 3rd, Monk BJ, Huang HQ, Grendys EC Jr, McMeekin DS, Sorosky J, et al. Clinical results and quality of life analysis for the MVAC combination (methotrexate, vinblastine, doxorubicin, and cisplatin) in carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Gynecologic Oncology 2006;100(3):537‐43. [PUBMED: 16216315] - PubMed
Malkasian 1981 {published data only}
-
- Malkasian GD Jr, Decker DG, Green SJ, Edmonson JH, Jefferies JA, Webb MJ. Treatment of recurrent and metastatic carcinoma of the cervix: comparison of doxorubicin with a combination of vincristine and 5‐fluorouracil. Gynecologic Oncology 1981;11(2):235‐9. [PUBMED: 7011913] - PubMed
McGuire 1989 {published data only}
-
- McGuire WP 3rd, Arseneau J, Blessing JA, DiSaia PJ, Hatch KD, Given FT Jr, et al. A randomized comparative trial of carboplatin and iproplatin in advanced squamous carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 1989;7(10):1462‐8. [PUBMED: 2674333] - PubMed
Monk 2008 {published data only}
Moore 2004 {published data only}
-
- McQuellon RP, Thaler HT, Cella D, Moore D. Quality of life outcomes from a randomised trial of cisplatin versus cisplatin plus paclitaxel in advanced cervical cancer: a Gynecologic Oncology Group study. Gynecologic Oncology 2006;101(2):296‐304. - PubMed
-
- Moore DH, Blessing JA, McQuellon RP, Thaler HT, Cella D, Benda J, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a gynecologic oncology group study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 2004;22(15):3113‐9. [PUBMED: 15284262] - PubMed
Moseley 1976 {published data only}
-
- Moseley HS, Sasaki T, McConnell DB, Merhoff GC, Wilson WL, Grage TB, et al. A randomized pilot study comparing two regimens in the treatment of squamous cell carcinoma. Journal of Surgical Oncology 1976;8(1):35‐42. [PUBMED: 55523] - PubMed
Mountzios 2009 {published data only}
-
- Mountzios G, Dimpoulos MA, Bamias A, Vourli G, Kalofonos H, Aravantinos G, et al. Randomise multicentre phase II trial of cisplatin and ifosfamide with or without paclitaxel in recurrent or metastatic carcinoma of the uterine cervix: a Hellenic Co‐operative Oncology Group (HeCOG) study. Annals of Oncology 2009;20:1362‐8. - PubMed
Omura 1978 {published data only}
-
- Omura GA, Shingleton HM, Creasman WT, Blessing JA, Boronow RC. Chemotherapy of gynecologic cancer with nitrosoureas: a randomized trial of CCNU and methyl‐CCNU in cancers of the cervix, corpus, vagina, and vulva. Cancer Treatment Reports 1978;62(5):833‐5. [PUBMED: 350400] - PubMed
Omura 1981 {published data only}
-
- Omura GA, Velez‐Garcia E, Birch R. Phase II randomized study of doxorubicin, vincristine, and 5‐FU versus cyclophosphamide in advanced squamous cell carcinoma of the cervix. Cancer Treatment Reports 1981;65(9‐10):901‐3. [PUBMED: 7196801] - PubMed
Omura 1997 {published data only}
-
- Omura GA, Blessing JA, Vaccarello L, Berman ML, Clarke‐Pearson DL, Mutch DG, et al. Randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 1997;15(1):165‐71. [PUBMED: 8996138] - PubMed
Palo 1976 {published data only}
-
- Palo GM, Bajetta E, Beretta G, Bonadonna G. Adriamycin plus bleomycin versus cyclophosphamide plus vincristine in advanced carcinoma of the uterine cervix. Tumori 1976;62(1):113‐22. [PUBMED: 65039] - PubMed
Pfeiffer 1998 {published data only}
-
- Pfeiffer P, Thomsen K, Bertelsen K. Teniposide or carboplatin in patients with recurrent or advanced cervical carcinoma. International Journal of Gynecological Cancer: Official Journal of the International Gynecological Cancer Society 1997;7(2):1.
-
- Thomsen TK, Pfeiffer P, Bertelsen K. Teniposide or carboplatin in patients with recurrent or advanced cervical carcinoma: a randomized phase II trial. International Journal of Gynecological Cancer 1998;8(4):310‐14.
Thigpen 1989 {published data only}
-
- Thigpen JT, Blessing JA, DiSaia PJ, Fowler WC Jr, Hatch KD. A randomized comparison of a rapid versus prolonged (24 hr) infusion of cisplatin in therapy of squamous cell carcinoma of the uterine cervix: a Gynecologic Oncology Group study. Gynecologic Oncology 1989;32(2):198‐202. [PUBMED: 2910782] - PubMed
Wallace 1978 {published data only}
-
- Wallace HJ Jr, Hreshchyshyn MM, Wilbanks GD, Boronow RC, Fowler WC Jr, Blessing JA. Comparison of the therapeutic effects of adriamycin alone versus adriamycin plus vincristine versus adriamycin plus cyclophosphamide in the treatment of advanced carcinoma of the cervix. Cancer Treatment Reports 1978;62(10):1435‐41. [PUBMED: 709549] - PubMed
Weiss 1992 {published data only}
-
- Weiss G, Liu P, O'Sullivan J, Alberts D, Brown T, Neefe J, Hutchins L. A randomised phase II trial of trimetrexate or didemnin for the treatment of metastatic or recurrent squamous carcinoma of the uterine cervix: a SWOG trial. Gynecologic Oncology 1992;45:303‐6. - PubMed
References to studies excluded from this review
Armstrong 2003 {published data only}
-
- Armstrong D, Blessing J, Rader J, Sorosky J. A randomised Phase II evaluation of bryostatin‐1 in persistent or recurrent squamous cell carcinoma of the cervix: a Gynaecological Oncology Group study. Investigational New Drugs 2003;21(4):453‐7. - PubMed
Baker 1978 {published data only}
-
- Baker LH, Opipari MI, Wilson H, Bottomley R, Coltman CA Jr. Mitomycin C, vincristine, and bleomycin therapy for advanced cervical cancer. Obstetrics and Gynecology 1978;52(2):146‐50. [PUBMED: 79991] - PubMed
Greggi 2000 {published data only}
-
- Greggi S, D'Agostino G, Smaniotto D, Genovesi D, Lorusso D, Scambia G. Gemcitabine is ineffective in recurrent, preirradiated cervical cancer. Gynecologic Oncology 2000; Vol. 78, issue 1:76‐7. [PUBMED: 10873416] - PubMed
Kumar 1998 {published data only}
-
- Kumar L, Pokharel YH, Kumar S, Singh R, Rath GK, Kochupillai V. Single agent versus combination chemotherapy in recurrent cervical cancer. The Journal of Obstetrics and Gynaecology Research 1998;24(6):401‐9. [PUBMED: 10063235] - PubMed
References to ongoing studies
Saito 2010 {published data only}
-
- Saito I, Kitagawa R, Fukuda H, Shibata T, Katsumata N, Konishi I, et al. A phase III trial of paclitaxel plus carboplatin versus paclitaxel plus cisplatin in stage IVB persistent or recurrent cervical cancer: a Gynecologic Cancer Study group/Japan Clinical Oncology Group study (JCOG0505). Japanese Journal of Clinical Oncology 2010;40(1):90‐3. - PubMed
Additional references
CTCAE 2006
-
- CTCAE. Common Terminology Criteria for Adverse Events V3.0, August 2006. http://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/... (accessed 5 August 2012).
de la Motte Rouge 2006
-
- Motte Rouge T, Pautier P, Hamy AS, Duvillard P, Bruna A, Castaigne D, et al. Medical treatment of metastatic or recurrent cancer of the cervix. Bull Cancer 2006;93(3):263‐70. - PubMed
Deeks 2001
-
- Deeks JJ, Altman DG, Bradburn MJ. Statistical methods for examining heterogeneity and combining results from several studies in meta‐analysis. In: Egger M, Davey Smith G, Altman DG editor(s). Systematic Reviews in Health Care: Meta‐Analysis in Context. 2nd Edition. London: BMJ Publication Group, 2001.
DerSimonian 1986
-
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. - PubMed
Eifel 2001
-
- Eifel PJ, Berek JS, Thigpen JT. Gynaecological Cancers. Section 2. Cancer of the cervix, vagina, vulva. In: DeVita VT, Hellman S, Rosenberg SA editor(s). Cancer: Principles and Practice of Oncology. 6. Vol. 2, Philadelphia: Lippincott‐Raven, 2001:1526‐1556.
EUROCARE‐3
-
- Sant M, Aareleid T, Berrino F, Bielska Lasota M, Carli PM, Faivre J, et al. EUROCARE‐3: survival of cancer patients diagnosed 1990‐94‐ results and commentary. Annals of Oncology 2003;14(Suppl 5):V61‐118. - PubMed
GLOBOCAN 2008
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C. Estimates of worldwide burden of cancer in 2008; GLOBOCAN. International Journal of Cancer 2010;127(12):2893‐917. - PubMed
Green 2005
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jemal 2008
-
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics 2008. CA: A Cancer Journal for Clinicians 2008;58(2):71‐96. - PubMed
Meta‐analysis Collaboration 2008
-
- Chemoradiotherapy for Cervical Cancer Meta‐analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: a systematic review and meta‐analysis of individual patient data from 18 randomized trials. Journal of Clinical Oncology 2008;26(35):5802‐12. - PMC - PubMed
Michiels 2005
-
- Michiels S, Piedbois P, Burdett S, Syz N, Stewart L, Pignon JP. Meta‐analysis when only the median survival times are known: a comparison with individual patient data results. International Journal of Technology Assessment in Healthcare 2005;21(1):119‐25. - PubMed
Miller 1981
-
- Miller AB, Hoogstraten B, Staquet M, Winkler A. Reporting results of cancer treatment. Cancer 1981;47:207‐14. - PubMed
Moore 2006
-
- Moore DH. Chemotherapy for recurrent cervical carcinoma. Current Opinions in Oncology 2006;18(5):516‐9. - PubMed
Moore 2007
-
- Moore K. A comparison of cisplatin/paclitaxel and carboplatin/paclitaxel in stage IV recurrent or persistent cervix cancer. Gynaecological Oncology 2007;105:299‐303. - PubMed
Mutch 2003
-
- Mutch D, Bloss J. Gemcitabine in cervical cancer. Gynaecological Oncology 2003;90(2 part 2):S8‐25. - PubMed
National Cancer Institute 2012
-
- National Cancer Institute. Cervical cancer, 2012. http://www.cancer.gov/cancertopics/types/cervical (accessed 5 August 2012).
Parmar 1998
-
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17:2815‐34. - PubMed
Pectasides 2008
-
- Pectasides D, Kamposioras K, Papaxoinis D, Pectasides E. Chemotherapy for recurrent cervical cancer. Cancer Treatment Reviews 2008;34(7):603‐13. - PubMed
RevMan 2011 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Tewari 2005
-
- Tewari KS, Monk BJ. Gynaecological group trials of chemotherapy for metastatic and recurrent cervical cancer. Current Oncology Reports 2005;7(6):419‐34. - PubMed
Tinker 2005
-
- Tinker A, Bhagat K, Swenerton K, Hoskins P. Carboplatin and paclitaxel for advanced and recurrent cervical carcinoma; the BCCA experience. Gynaecological Oncology 2005;98(1):54‐8. - PubMed
Weiss 1990
-
- Weiss GR, Green S, Hannigan EV, Boutselis JG, Surwit EA, Wallace DL, et al. A Phase II trial of carboplatin for recurrent or metastatic squamous carcinoma of the uterine cervix. A SWOG study. Gynaecological Oncology 1990;39(3):332‐6. - PubMed
Willmott 2009
-
- Willmott LJ, Monk B. Cervical cancer therapy. Expert Review of Anticancer Therapy 2009;9(7):895‐903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

