Laminins in basement membrane assembly
- PMID: 23076216
- PMCID: PMC3544787
- DOI: 10.4161/cam.21831
Laminins in basement membrane assembly
Abstract
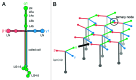
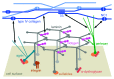
The heterotrimeric laminins are a defining component of all basement membranes and self-assemble into a cell-associated network. The three short arms of the cross-shaped laminin molecule form the network nodes, with a strict requirement for one α, one β and one γ arm. The globular domain at the end of the long arm binds to cellular receptors, including integrins, α-dystroglycan, heparan sulfates and sulfated glycolipids. Collateral anchorage of the laminin network is provided by the proteoglycans perlecan and agrin. A second network is then formed by type IV collagen, which interacts with the laminin network through the heparan sulfate chains of perlecan and agrin and additional linkage by nidogen. This maturation of basement membranes becomes essential at later stages of embryo development.
Figures

Similar articles
-
Scaffold-forming and Adhesive Contributions of Synthetic Laminin-binding Proteins to Basement Membrane Assembly.J Biol Chem. 2009 Mar 27;284(13):8984-94. doi: 10.1074/jbc.M809719200. Epub 2009 Feb 2. J Biol Chem. 2009. PMID: 19189961 Free PMC article.
-
Integrating Activities of Laminins that Drive Basement Membrane Assembly and Function.Curr Top Membr. 2015;76:1-30. doi: 10.1016/bs.ctm.2015.05.001. Epub 2015 Jun 25. Curr Top Membr. 2015. PMID: 26610910 Review.
-
Linker molecules between laminins and dystroglycan ameliorate laminin-alpha2-deficient muscular dystrophy at all disease stages.J Cell Biol. 2007 Mar 26;176(7):979-93. doi: 10.1083/jcb.200611152. J Cell Biol. 2007. PMID: 17389231 Free PMC article.
-
Basement membrane composition in the early mouse embryo day 7.Dev Dyn. 2005 Jul;233(3):1140-8. doi: 10.1002/dvdy.20425. Dev Dyn. 2005. PMID: 15895400
-
The nature and biology of basement membranes.Matrix Biol. 2017 Jan;57-58:1-11. doi: 10.1016/j.matbio.2016.12.009. Epub 2016 Dec 28. Matrix Biol. 2017. PMID: 28040522 Free PMC article. Review.
Cited by
-
Limb girdle muscular dystrophy due to LAMA2 gene mutations: new mutations expand the clinical spectrum of a still challenging diagnosis.Acta Myol. 2020 Jun 1;39(2):67-82. doi: 10.36185/2532-1900-009. eCollection 2020 Jun. Acta Myol. 2020. PMID: 32904964 Free PMC article.
-
Insights into the Tumor Microenvironment-Components, Functions and Therapeutics.Int J Mol Sci. 2023 Dec 15;24(24):17536. doi: 10.3390/ijms242417536. Int J Mol Sci. 2023. PMID: 38139365 Free PMC article. Review.
-
Hanging on for the ride: adhesion to the extracellular matrix mediates cellular responses in skeletal muscle morphogenesis and disease.Dev Biol. 2015 May 1;401(1):75-91. doi: 10.1016/j.ydbio.2015.01.002. Epub 2015 Jan 12. Dev Biol. 2015. PMID: 25592225 Free PMC article. Review.
-
Mechanochemical Signaling of the Extracellular Matrix in Epithelial-Mesenchymal Transition.Front Cell Dev Biol. 2019 Jul 19;7:135. doi: 10.3389/fcell.2019.00135. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31380370 Free PMC article. Review.
-
Basement Membranes in Development and Disease.Curr Top Dev Biol. 2018;130:143-191. doi: 10.1016/bs.ctdb.2018.02.005. Epub 2018 Mar 31. Curr Top Dev Biol. 2018. PMID: 29853176 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
