Oroxylin-A rescues LPS-induced acute lung injury via regulation of NF-κB signaling pathway in rodents
- PMID: 23071799
- PMCID: PMC3468516
- DOI: 10.1371/journal.pone.0047403
Oroxylin-A rescues LPS-induced acute lung injury via regulation of NF-κB signaling pathway in rodents
Abstract
Background and purpose: Successful drug treatment for sepsis-related acute lung injury (ALI) remains a major clinical problem. This study was designed to assess the beneficial effects of post-treatment of oroxylin A (OroA), a flavonoid, in ameliorating lipopolysaccharides (LPS)-induced lung inflammation and fatality.
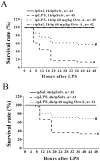
Experimental approach: Rats were injected with LPS (10 mg/kg, iv) to induce ALI, and OroA was given (15 mg/kg, iv) 1 hr or 6 hrs after LPS challenge. Twenty four hrs after LPS challenge, biochemical changes in the blood and lung tissues, and morphological/histological alterations in the lung associated with inflammation and injury were examined. Therapeutic effect of OroA was assessed by measuring the survival rate in endotoxemic mice.
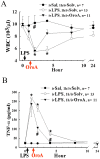
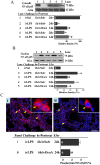
Key results: LPS (10 mg/kg, iv) significantly altered WBC counts, elevated plasma tumor necrosis factor (TNF)-α and nitric oxide (NO), increased pulmonary edema, thickened alveolar septa, and decreased survival rate. These changes were ameliorated by OroA (15 mg/kg, iv) administered 1 hr or 6 hrs after LPS challenge. This post-treatment also significantly attenuated LPS-induced activation of nuclear factor-κB (NF-κB) and the release of high mobility group box 1 (HMGB1) in lung tissues. Furthermore, post-treatment with OroA (60 mg/kg, ip) administered 1 hr or 6 hrs after LPS challenge in mice significantly increased survival rate.
Conclusion and implication: OroA administered after induction of ALI by LPS significantly prevent and revere lung tissues injuries with increased survival rate. Positive post-treatment effects of OroA suggest that OroA is a potentially useful candidate for managing lung inflammation in LPS-induced endotoxemia and septic shock.
Conflict of interest statement
Figures




Similar articles
-
Induction of endothelium-dependent constriction of mesenteric arteries in endotoxemic hypotensive shock.Br J Pharmacol. 2016 Apr;173(7):1179-95. doi: 10.1111/bph.13415. Epub 2016 Mar 6. Br J Pharmacol. 2016. PMID: 26694894 Free PMC article.
-
Kegan Liyan oral liquid ameliorates lipopolysaccharide-induced acute lung injury through inhibition of TLR4-mediated NF-κB signaling pathway and MMP-9 expression.J Ethnopharmacol. 2016 Jun 20;186:91-102. doi: 10.1016/j.jep.2016.03.057. Epub 2016 Mar 30. J Ethnopharmacol. 2016. PMID: 27036629
-
Cavidine Ameliorates Lipopolysaccharide-Induced Acute Lung Injury via NF-κB Signaling Pathway in vivo and in vitro.Inflammation. 2017 Aug;40(4):1111-1122. doi: 10.1007/s10753-017-0553-1. Inflammation. 2017. PMID: 28365871
-
Tetrahydroberberrubine attenuates lipopolysaccharide-induced acute lung injury by down-regulating MAPK, AKT, and NF-κB signaling pathways.Biomed Pharmacother. 2016 Aug;82:489-97. doi: 10.1016/j.biopha.2016.05.025. Epub 2016 Jun 2. Biomed Pharmacother. 2016. PMID: 27470389
-
[Effect of growth hormone on acute lung injury].Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2005 Sep;17(9):523-6. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue. 2005. PMID: 16146594 Chinese.
Cited by
-
Effect of ERK1/2 signaling pathway in electro-acupuncture mediated up-regulation of heme oxygenase-1 in lungs of rabbits with endotoxic shock.Med Sci Monit. 2014 Aug 16;20:1452-60. doi: 10.12659/MSM.890736. Med Sci Monit. 2014. PMID: 25139460 Free PMC article.
-
Potential effects of medicinal plants and secondary metabolites on acute lung injury.Biomed Res Int. 2013;2013:576479. doi: 10.1155/2013/576479. Epub 2013 Oct 9. Biomed Res Int. 2013. PMID: 24224172 Free PMC article. Review.
-
HMGB1 in health and disease.Mol Aspects Med. 2014 Dec;40:1-116. doi: 10.1016/j.mam.2014.05.001. Epub 2014 Jul 8. Mol Aspects Med. 2014. PMID: 25010388 Free PMC article. Review.
-
Terretonin as a New Protective Agent against Sepsis-Induced Acute Lung Injury: Impact on SIRT1/Nrf2/NF-κBp65/NLRP3 Signaling.Biology (Basel). 2021 Nov 22;10(11):1219. doi: 10.3390/biology10111219. Biology (Basel). 2021. PMID: 34827212 Free PMC article.
-
Oroxylin A inhibits colitis by inactivating NLRP3 inflammasome.Oncotarget. 2017 Jul 22;8(35):58903-58917. doi: 10.18632/oncotarget.19440. eCollection 2017 Aug 29. Oncotarget. 2017. PMID: 28938606 Free PMC article.
References
-
- Dreyfuss D, Ricard JD (2005) Acute lung injury and bacterial infection. Clin Chest Med 26: 105–112. - PubMed
-
- Baumgarten G, Knuefermann P, Wrigge H, Putensen C, Stapel H, et al. (2006) Role of Toll-like receptor 4 for the pathogenesis of acute lung injury in Gram-negative sepsis. Eur J Anaesthesiol 23: 1041–1048. - PubMed
-
- Jiang Y, Xu J, Zhou C, Wu Z, Zhong S, et al. (2005) Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am J Respir Crit Care Med 171: 850–857. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

