MOSAIC: a multiscale model of osteogenesis and sprouting angiogenesis with lateral inhibition of endothelial cells
- PMID: 23071433
- PMCID: PMC3469420
- DOI: 10.1371/journal.pcbi.1002724
MOSAIC: a multiscale model of osteogenesis and sprouting angiogenesis with lateral inhibition of endothelial cells
Erratum in
- PLoS Comput Biol. 2013 Mar;9(3). doi: 10.1371/annotation/38264a13-d4b5-49cd-b54e-47330bb19fe9
Abstract
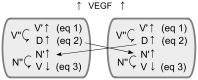
The healing of a fracture depends largely on the development of a new blood vessel network (angiogenesis) in the callus. During angiogenesis tip cells lead the developing sprout in response to extracellular signals, amongst which vascular endothelial growth factor (VEGF) is critical. In order to ensure a correct development of the vasculature, the balance between stalk and tip cell phenotypes must be tightly controlled, which is primarily achieved by the Dll4-Notch1 signaling pathway. This study presents a novel multiscale model of osteogenesis and sprouting angiogenesis, incorporating lateral inhibition of endothelial cells (further denoted MOSAIC model) through Dll4-Notch1 signaling, and applies it to fracture healing. The MOSAIC model correctly predicted the bone regeneration process and recapitulated many experimentally observed aspects of tip cell selection: the salt and pepper pattern seen for cell fates, an increased tip cell density due to the loss of Dll4 and an excessive number of tip cells in high VEGF environments. When VEGF concentration was even further increased, the MOSAIC model predicted the absence of a vascular network and fracture healing, thereby leading to a non-union, which is a direct consequence of the mutual inhibition of neighboring cells through Dll4-Notch1 signaling. This result was not retrieved for a more phenomenological model that only considers extracellular signals for tip cell migration, which illustrates the importance of implementing the actual signaling pathway rather than phenomenological rules. Finally, the MOSAIC model demonstrated the importance of a proper criterion for tip cell selection and the need for experimental data to further explore this. In conclusion, this study demonstrates that the MOSAIC model creates enhanced capabilities for investigating the influence of molecular mechanisms on angiogenesis and its relation to bone formation in a more mechanistic way and across different time and spatial scales.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
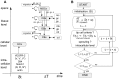
 : vector of the continuous variables, t: time, δt: time step of the inner loop, Δt: time step of the outer loop, cv: endothelial cells).
: vector of the continuous variables, t: time, δt: time step of the inner loop, Δt: time step of the outer loop, cv: endothelial cells).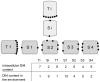
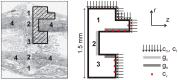
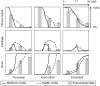



Similar articles
-
Delta-like ligand 4 regulates vascular endothelial growth factor receptor 2-driven luteal angiogenesis through induction of a tip/stalk phenotype in proliferating endothelial cells.Fertil Steril. 2013 Dec;100(6):1768-76.e1. doi: 10.1016/j.fertnstert.2013.08.034. Epub 2013 Sep 26. Fertil Steril. 2013. PMID: 24074756
-
Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis.Nature. 2007 Feb 15;445(7129):776-80. doi: 10.1038/nature05571. Epub 2007 Jan 28. Nature. 2007. PMID: 17259973
-
Tip cell overtaking occurs as a side effect of sprouting in computational models of angiogenesis.BMC Syst Biol. 2015 Nov 21;9:86. doi: 10.1186/s12918-015-0230-7. BMC Syst Biol. 2015. PMID: 26589386 Free PMC article.
-
VEGFRs and Notch: a dynamic collaboration in vascular patterning.Biochem Soc Trans. 2009 Dec;37(Pt 6):1233-6. doi: 10.1042/BST0371233. Biochem Soc Trans. 2009. PMID: 19909253 Review.
-
Ligand-dependent Notch signaling in vascular formation.Adv Exp Med Biol. 2012;727:210-22. doi: 10.1007/978-1-4614-0899-4_16. Adv Exp Med Biol. 2012. PMID: 22399350 Review.
Cited by
-
On the role of mechanical signals on sprouting angiogenesis through computer modeling approaches.Biomech Model Mechanobiol. 2022 Dec;21(6):1623-1640. doi: 10.1007/s10237-022-01648-4. Epub 2022 Nov 17. Biomech Model Mechanobiol. 2022. PMID: 36394779 Free PMC article. Review.
-
Review on experiment-based two- and three-dimensional models for wound healing.Interface Focus. 2016 Oct 6;6(5):20160038. doi: 10.1098/rsfs.2016.0038. Interface Focus. 2016. PMID: 27708762 Free PMC article. Review.
-
Three-dimensional computational model simulating the fracture healing process with both biphasic poroelastic finite element analysis and fuzzy logic control.Sci Rep. 2018 Apr 30;8(1):6744. doi: 10.1038/s41598-018-25229-7. Sci Rep. 2018. PMID: 29712979 Free PMC article.
-
In silico bone mechanobiology: modeling a multifaceted biological system.Wiley Interdiscip Rev Syst Biol Med. 2016 Nov;8(6):485-505. doi: 10.1002/wsbm.1356. Epub 2016 Sep 7. Wiley Interdiscip Rev Syst Biol Med. 2016. PMID: 27600060 Free PMC article. Review.
-
Stromal cells and stem cells in clinical bone regeneration.Nat Rev Endocrinol. 2015 Mar;11(3):140-50. doi: 10.1038/nrendo.2014.234. Epub 2015 Jan 6. Nat Rev Endocrinol. 2015. PMID: 25560703 Free PMC article. Review.
References
-
- Taguchi K, Ogawa R, Migita M, Hanawa H, Ito H, et al. (2005) The role of bone marrow-derived cells in bone fracture repair in a green fluorescent protein chimeric mouse model. Biochem Biophys Res Commun 331: 31–36. - PubMed
-
- Carmeliet P, De Smet F, Loges S, Mazzone M (2009) Branching morphogenesis and antiangiogenesis candidates: tip cells lead the way. Nat Rev Clin Oncol 6: 315–326. - PubMed
-
- Bentley K, Gerhardt H, Bates PA (2008) Agent-based simulation of notch-mediated tip cell selection in angiogenic sprout initialisation. J Theor Biol 250: 25–36 S0022-5193(07)00443-2 [pii];10.1016/j.jtbi.2007.09.015 [doi]. - PubMed
-
- De Smet F, Segura I, De Bock K, Hohensinner PJ, Carmeliet P (2009) Mechanisms of Vessel Branching Filopodia on Endothelial Tip Cells Lead the Way. Arterioscler Thromb Vasc Biol 29: 639–649. - PubMed
-
- Hellstrom M, Phng LK, Hofmann JJ, Wallgard E, Coultas L, et al. (2007) Dll4 signalling through Notch1 regulates formation of tip cells during angiogenesis. Nature 445: 776–780. - PubMed

