Serine phosphorylation by SYK is critical for nuclear localization and transcription factor function of Ikaros
- PMID: 23071339
- PMCID: PMC3497833
- DOI: 10.1073/pnas.1209828109
Serine phosphorylation by SYK is critical for nuclear localization and transcription factor function of Ikaros
Abstract
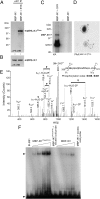
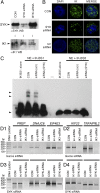
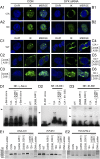
Ikaros is a zinc finger-containing DNA-binding protein that plays a pivotal role in immune homeostasis through transcriptional regulation of the earliest stages of lymphocyte ontogeny and differentiation. Functional deficiency of Ikaros has been implicated in the pathogenesis of acute lymphoblastic leukemia, the most common form of childhood cancer. Therefore, a stringent regulation of Ikaros activity is considered of paramount importance, but the operative molecular mechanisms responsible for its regulation remain largely unknown. Here we provide multifaceted genetic and biochemical evidence for a previously unknown function of spleen tyrosine kinase (SYK) as a partner and posttranslational regulator of Ikaros. We demonstrate that SYK phoshorylates Ikaros at unique C-terminal serine phosphorylation sites S358 and S361, thereby augmenting its nuclear localization and sequence-specific DNA binding activity. Mechanistically, we establish that SYK-induced Ikaros activation is essential for its nuclear localization and optimal transcription factor function.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Regulatory phosphorylation of Ikaros by Bruton's tyrosine kinase.PLoS One. 2013 Aug 19;8(8):e71302. doi: 10.1371/journal.pone.0071302. eCollection 2013. PLoS One. 2013. PMID: 23977012 Free PMC article.
-
Transcriptional Regulation of PIK3CD and PIKFYVE in T-Cell Acute Lymphoblastic Leukemia by IKAROS and Protein Kinase CK2.Int J Mol Sci. 2021 Jan 15;22(2):819. doi: 10.3390/ijms22020819. Int J Mol Sci. 2021. PMID: 33467550 Free PMC article.
-
IRF4 modulates the response to BCR activation in chronic lymphocytic leukemia regulating IKAROS and SYK.Leukemia. 2021 May;35(5):1330-1343. doi: 10.1038/s41375-021-01178-5. Epub 2021 Feb 23. Leukemia. 2021. PMID: 33623139
-
Regulation of cellular proliferation in acute lymphoblastic leukemia by Casein Kinase II (CK2) and Ikaros.Adv Biol Regul. 2017 Jan;63:71-80. doi: 10.1016/j.jbior.2016.09.003. Epub 2016 Sep 18. Adv Biol Regul. 2017. PMID: 27666503 Free PMC article. Review.
-
Transcriptional Regulation of Genes by Ikaros Tumor Suppressor in Acute Lymphoblastic Leukemia.Int J Mol Sci. 2020 Feb 18;21(4):1377. doi: 10.3390/ijms21041377. Int J Mol Sci. 2020. PMID: 32085659 Free PMC article. Review.
Cited by
-
Calling in SYK: SYK's dual role as a tumor promoter and tumor suppressor in cancer.Biochim Biophys Acta. 2015 Jan;1853(1):254-63. doi: 10.1016/j.bbamcr.2014.10.022. Epub 2014 Nov 4. Biochim Biophys Acta. 2015. PMID: 25447675 Free PMC article. Review.
-
Recombinant human CD19L-sTRAIL effectively targets B cell precursor acute lymphoblastic leukemia.J Clin Invest. 2015 Mar 2;125(3):1006-18. doi: 10.1172/JCI76610. Epub 2015 Jan 26. J Clin Invest. 2015. PMID: 25621496 Free PMC article.
-
IKAROS: from chromatin organization to transcriptional elongation control.Cell Death Differ. 2025 Jan;32(1):37-55. doi: 10.1038/s41418-023-01212-2. Epub 2023 Aug 24. Cell Death Differ. 2025. PMID: 37620540 Review.
-
Ikaros is absolutely required for pre-B cell differentiation by attenuating IL-7 signals.J Exp Med. 2013 Dec 16;210(13):2823-32. doi: 10.1084/jem.20131735. Epub 2013 Dec 2. J Exp Med. 2013. PMID: 24297995 Free PMC article.
-
The many faces of IKZF1 in B-cell precursor acute lymphoblastic leukemia.Haematologica. 2018 Apr;103(4):565-574. doi: 10.3324/haematol.2017.185603. Epub 2018 Mar 8. Haematologica. 2018. PMID: 29519871 Free PMC article. Review.
References
-
- Thompson EC, et al. Ikaros DNA-binding proteins as integral components of B cell developmental-stage-specific regulatory circuits. Immunity. 2007;26(3):335–344. - PubMed
-
- Merkenschlager M. Ikaros in immune receptor signaling, lymphocyte differentiation, and function. FEBS Lett. 2010;584(24):4910–4914. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

