Antibodies anti-CagA cross-react with trophoblast cells: a risk factor for pre-eclampsia?
- PMID: 23066738
- PMCID: PMC3739447
- DOI: 10.1111/j.1523-5378.2012.00966.x
Antibodies anti-CagA cross-react with trophoblast cells: a risk factor for pre-eclampsia?
Abstract
Background: Previous studies reported an epidemiological association between CagA-positive H. pylori strains and pre-eclampsia. As antibodies anti-CagA cross-react with endothelial cells and trophoblast cells show an endothelial phenotypic profile, we hypothesized that anti-CagA antibodies may recognize antigens of cytotrophoblast cells, thus impairing their function.
Materials and methods: Placenta samples were obtained from healthy women. Cytotrophoblast cells were cultured in a medium containing increasing concentration of polyclonal anti-CagA antibodies. Binding of anti-CagA antibodies to cytotrophoblast cells was evaluated by cell ELISA and immunofluorescence assay. Invasive potential of those cells was assessed by an invasion culture system and by measuring of MMP-2. Protein sequencing was performed on antigens precipitated by anti-CagA antibodies. Measurement of phosphorylated ERK expression and NF-kB DNA-binding activity in trophoblast cells incubated with anti-CagA or irrelevant antibodies was also performed.
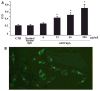
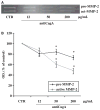
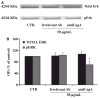
Results: Anti-CagA antibodies recognized β-actin of cytotrophoblast cells, showing a dose-dependent binding. Incubation of cytotrophoblast cells with increasing doses of anti-CagA antibodies significantly reduced their invasiveness and determined a significant decrease in phosphorylated ERK expression and a reduced NF-kB translocation activity.
Conclusions: This study shows that anti-CagA antibodies recognize β-actin of cytotrophoblast cells, reducing their invasiveness ability, possibly giving a biological explanation for the epidemiological association.
© 2012 Blackwell Publishing Ltd.
Conflict of interest statement
Figures
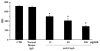


Similar articles
-
Cross-reactivity of anti-CagA antibodies with vascular wall antigens: possible pathogenic link between Helicobacter pylori infection and atherosclerosis.Circulation. 2002 Jul 23;106(4):430-4. doi: 10.1161/01.cir.0000024100.90140.19. Circulation. 2002. PMID: 12135941
-
[Production of a recombinant CagA protein for the detection of Helicobacter pylori CagA antibodies].Mikrobiyol Bul. 2014 Jul;48(3):402-12. doi: 10.5578/mb.7642. Mikrobiyol Bul. 2014. PMID: 25052106 Turkish.
-
Detection of Anti-CagA Antibodies in Sera of Helicobacter pylori-Infected Patients Using an Immunochromatographic Test Strip.J Chromatogr Sci. 2020 Apr 22;58(3):217-222. doi: 10.1093/chromsci/bmz093. J Chromatogr Sci. 2020. PMID: 31812997
-
H pylori infection and systemic antibodies to CagA and heat shock protein 60 in patients with coronary heart disease.World J Gastroenterol. 2006 Dec 28;12(48):7815-20. doi: 10.3748/wjg.v12.i48.7815. World J Gastroenterol. 2006. PMID: 17203526 Free PMC article.
-
Systematic analysis of phosphotyrosine antibodies recognizing single phosphorylated EPIYA-motifs in CagA of East Asian-type Helicobacter pylori strains.BMC Microbiol. 2016 Sep 2;16(1):201. doi: 10.1186/s12866-016-0820-6. BMC Microbiol. 2016. PMID: 27590005 Free PMC article.
Cited by
-
The association of Helicobacter pylori with adverse pregnancy outcomes in three European birth cohorts.BMC Pregnancy Childbirth. 2024 Nov 12;24(1):745. doi: 10.1186/s12884-024-06901-5. BMC Pregnancy Childbirth. 2024. PMID: 39533217 Free PMC article.
-
Beyond the stomach: an updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment.World J Gastroenterol. 2014 Sep 28;20(36):12781-808. doi: 10.3748/wjg.v20.i36.12781. World J Gastroenterol. 2014. PMID: 25278678 Free PMC article. Review.
-
Causal associations between Helicobacter pylori infection and pregnancy and neonatal outcomes: a two-sample Mendelian randomization study.Front Cell Infect Microbiol. 2024 Mar 14;14:1343499. doi: 10.3389/fcimb.2024.1343499. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38558850 Free PMC article.
-
Relationship Between Helicobacter pylori Infection, Serum Vitamin D3 Level and Spontaneous Abortion.Int J Gen Med. 2020 Jul 28;13:469-476. doi: 10.2147/IJGM.S251075. eCollection 2020. Int J Gen Med. 2020. PMID: 32801841 Free PMC article.
-
Association of Serum Hepcidin With Preeclampsia: A Systematic Review and Meta-Analysis.Cureus. 2022 Jul 9;14(7):e26699. doi: 10.7759/cureus.26699. eCollection 2022 Jul. Cureus. 2022. PMID: 35959172 Free PMC article. Review.
References
-
- Shenoy V, Kanasaki K, Kalluri R. Pre-eclampsia: connecting angiogenic and metabolic pathways. Trends Endocrinol Metab. 2010;21:529–36. - PubMed
-
- Steegers EA, von Dadelszen P, Duvekot JJ, et al. Pre-eclampsia. Lancet. 2010;376:631–44. - PubMed
-
- James JL, Whitley GS, Cartwright JE. Pre-eclampsia: fitting together the placental, immune and cardiovascular pieces. J Pathol. 2010;221:363–78. - PubMed
-
- Macey MG, Bevan S, Alam S, et al. Platelet activation and endogenous thrombin potential in pre-eclampsia. Thromb Res. 2010;125:e76–81. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

