The role of oxygen during fracture healing
- PMID: 23063782
- PMCID: PMC4827706
- DOI: 10.1016/j.bone.2012.09.037
The role of oxygen during fracture healing
Abstract
Oxygen affects the activity of multiple skeletogenic cells and is involved in many processes that are important for fracture healing. However, the role of oxygen in fracture healing has not been fully studied. Here we systematically examine the effects of oxygen tension on fracture healing and test the ability of hyperoxia to rescue healing defects in a mouse model of ischemic fracture healing. Mice with tibia fracture were housed in custom-built gas chambers and groups breathed a constant atmosphere of 13% oxygen (hypoxia), 21% oxygen (normoxia), or 50% oxygen (hyperoxia). The influx of inflammatory cells to the fracture site, stem cell differentiation, tissue vascularization, and fracture healing were analyzed. In addition, the efficacy of hyperoxia (50% oxygen) as a treatment regimen for fracture nonunion was tested. Hypoxic animals had decreased tissue vascularity, decreased bone formation, and delayed callus remodeling. Hyperoxia increased tissue vascularization, altered fracture healing in un-complicated fractures, and improved bone repair in ischemia-induced delayed fracture union. However, neither hypoxia nor hyperoxia significantly altered chondrogenesis or osteogenesis during early stages of fracture healing, and infiltration of macrophages and neutrophils was not affected by environmental oxygen after bone injury. In conclusion, our results indicate that environmental oxygen levels affect tissue vascularization and fracture healing, and that providing oxygen when fractures are accompanied by ischemia may be beneficial.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures


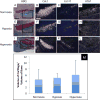

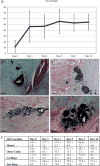
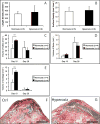
Comment in
-
Oxygen-generating scaffolds: a new strategy for bone tissue engineering.Bone. 2013 Nov;57(1):322-3. doi: 10.1016/j.bone.2013.07.034. Epub 2013 Jul 26. Bone. 2013. PMID: 23891852 No abstract available.
-
Beneficial effects of oxygen- and lactate-production in scaffold designs.Bone. 2013 Nov;57(1):324. doi: 10.1016/j.bone.2013.07.031. Epub 2013 Jul 26. Bone. 2013. PMID: 23895996 No abstract available.
Similar articles
-
Ischemia leads to delayed union during fracture healing: a mouse model.J Orthop Res. 2007 Jan;25(1):51-61. doi: 10.1002/jor.20264. J Orthop Res. 2007. PMID: 17019699 Free PMC article.
-
Mobilization of endothelial progenitor cells in fracture healing and distraction osteogenesis.Bone. 2008 May;42(5):932-41. doi: 10.1016/j.bone.2008.01.007. Epub 2008 Jan 26. Bone. 2008. PMID: 18326482
-
Temporal and spatial vascularization patterns of unions and nonunions: role of vascular endothelial growth factor and bone morphogenetic proteins.J Bone Joint Surg Am. 2012 Jan 4;94(1):49-58. doi: 10.2106/JBJS.J.00795. J Bone Joint Surg Am. 2012. PMID: 22218382
-
Osteogenic Differentiation of Periosteal Cells During Fracture Healing.J Cell Physiol. 2017 May;232(5):913-921. doi: 10.1002/jcp.25641. Epub 2016 Oct 26. J Cell Physiol. 2017. PMID: 27731505 Free PMC article. Review.
-
Stem Cell Interventions for Bone Healing: Fractures and Osteoporosis.Curr Stem Cell Res Ther. 2018;13(5):369-377. doi: 10.2174/1574888X13666180410160511. Curr Stem Cell Res Ther. 2018. PMID: 29637866 Review.
Cited by
-
The effect of bone marrow concentrate and hyperbaric oxygen therapy on bone repair.J Mater Sci Mater Med. 2015 Jan;26(1):5331. doi: 10.1007/s10856-014-5331-0. Epub 2015 Jan 11. J Mater Sci Mater Med. 2015. PMID: 25577213
-
The multifaceted role of the vasculature in endochondral fracture repair.Front Endocrinol (Lausanne). 2015 Feb 5;6:4. doi: 10.3389/fendo.2015.00004. eCollection 2015. Front Endocrinol (Lausanne). 2015. PMID: 25699016 Free PMC article. Review.
-
Bacterial Hypoxic Responses Revealed as Critical Determinants of the Host-Pathogen Outcome by TnSeq Analysis of Staphylococcus aureus Invasive Infection.PLoS Pathog. 2015 Dec 18;11(12):e1005341. doi: 10.1371/journal.ppat.1005341. eCollection 2015 Dec. PLoS Pathog. 2015. PMID: 26684646 Free PMC article.
-
Stimulating angiogenesis mitigates the unloading-induced reduction in osteogenesis in early-stage bone repair in rats.Physiol Rep. 2015 Mar;3(3):e12335. doi: 10.14814/phy2.12335. Physiol Rep. 2015. PMID: 25780087 Free PMC article.
-
Human bone marrow-derived mesenchymal stem cells display enhanced clonogenicity but impaired differentiation with hypoxic preconditioning.Stem Cells Transl Med. 2014 Feb;3(2):241-54. doi: 10.5966/sctm.2013-0079. Epub 2014 Jan 16. Stem Cells Transl Med. 2014. PMID: 24436440 Free PMC article.
References
-
- Dickson KF, Katzman S, Paiement G. The importance of the blood supply in the healing of tibial fractures. Contemporary orthopaedics. 1995;30:489–93. - PubMed
-
- Brinker MR, Bailey DE., Jr Fracture healing in tibia fractures with an associated vascular injury. The Journal of trauma. 1997;42:11–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

