TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities
- PMID: 23063463
- PMCID: PMC3508263
- DOI: 10.1016/j.cellsig.2012.10.003
TGF-β signaling in tissue fibrosis: redox controls, target genes and therapeutic opportunities
Abstract
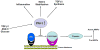
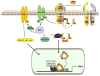
During development of TGF-β1-initiated fibroproliferative disorders, NADPH oxidases (NOX family members) generate reactive oxygen species (ROS) resulting in downstream transcription of a subset genes encoding matrix structural elements and profibrotic factors. Prominent among the repertoire of disease-implicated genes is the TGF-β1 target gene encoding the potent profibrotic matricellular protein plasminogen activator inhibitor-1 (PAI-1 or SERPINE1). PAI-1 is the major physiologic inhibitor of the plasmin-based pericellular cascade and a causative factor in the development of vascular thrombotic and fibroproliferative disorders. ROS generation in response to TGF-β1 stimulation is rapid and precedes PAI-1 induction; engagement of non-SMAD (e.g., EGFR, Src kinase, MAP kinases, p53) and SMAD2/3 pathways are both required for PAI-1 expression and are ROS-dependent. Recent findings suggest a novel role for p53 in TGF-β1-induced PAI-1 transcription that involves ROS generation and p53/SMAD interactions. Targeting ROS and ROS-activated cellular events is likely to have therapeutic implications in the management of fibrotic disorders, particularly in the context of prolonged TGF-β1 signaling.
Copyright © 2012 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures
Similar articles
-
Induction of renal fibrotic genes by TGF-β1 requires EGFR activation, p53 and reactive oxygen species.Cell Signal. 2013 Nov;25(11):2198-209. doi: 10.1016/j.cellsig.2013.07.007. Epub 2013 Jul 18. Cell Signal. 2013. PMID: 23872073
-
Redox control of p53 in the transcriptional regulation of TGF-β1 target genes through SMAD cooperativity.Cell Signal. 2014 Jul;26(7):1427-36. doi: 10.1016/j.cellsig.2014.02.017. Epub 2014 Mar 5. Cell Signal. 2014. PMID: 24613410 Free PMC article.
-
Redox-induced Src kinase and caveolin-1 signaling in TGF-β1-initiated SMAD2/3 activation and PAI-1 expression.PLoS One. 2011;6(7):e22896. doi: 10.1371/journal.pone.0022896. Epub 2011 Jul 28. PLoS One. 2011. PMID: 21829547 Free PMC article.
-
Integration of non-SMAD and SMAD signaling in TGF-beta1-induced plasminogen activator inhibitor type-1 gene expression in vascular smooth muscle cells.Thromb Haemost. 2008 Dec;100(6):976-83. Thromb Haemost. 2008. PMID: 19132220 Free PMC article. Review.
-
The TGF-β1/p53/PAI-1 Signaling Axis in Vascular Senescence: Role of Caveolin-1.Biomolecules. 2019 Aug 3;9(8):341. doi: 10.3390/biom9080341. Biomolecules. 2019. PMID: 31382626 Free PMC article. Review.
Cited by
-
Proteinase-activated receptor 2 promotes TGF-β-dependent cell motility in pancreatic cancer cells by sustaining expression of the TGF-β type I receptor ALK5.Oncotarget. 2016 Jul 5;7(27):41095-41109. doi: 10.18632/oncotarget.9600. Oncotarget. 2016. PMID: 27248167 Free PMC article.
-
Preventive Effect of 3,3'-dimethoxy-4,4'-dihydroxy-stilbene Triazole on Pulmonary Fibrosis through Inhibition of Inflammation and Down-regulation of TGF-b Signaling Pathway.Dokl Biochem Biophys. 2024 Dec;519(1):571-579. doi: 10.1134/S1607672924600350. Epub 2024 Oct 11. Dokl Biochem Biophys. 2024. PMID: 39400768
-
Can Nrf2 Modulate the Development of Intestinal Fibrosis and Cancer in Inflammatory Bowel Disease?Int J Mol Sci. 2019 Aug 20;20(16):4061. doi: 10.3390/ijms20164061. Int J Mol Sci. 2019. PMID: 31434263 Free PMC article. Review.
-
Extracellular matrix stiffness-The central cue for skin fibrosis.Front Mol Biosci. 2023 Mar 8;10:1132353. doi: 10.3389/fmolb.2023.1132353. eCollection 2023. Front Mol Biosci. 2023. PMID: 36968277 Free PMC article. Review.
-
TRPV1 potentiates TGFβ-induction of corneal myofibroblast development through an oxidative stress-mediated p38-SMAD2 signaling loop.PLoS One. 2013 Oct 2;8(10):e77300. doi: 10.1371/journal.pone.0077300. eCollection 2013. PLoS One. 2013. PMID: 24098582 Free PMC article.
References
-
- Chan EC, Jiang F, Peshavariya HM, Dusting GJ. Regulation of cell proliferation by NADPH oxidase-mediated signaling: potential roles in tissue repair, regenerative medicine and tissue engineering. Pharmacol Therap. 2009;122:97–108. - PubMed
-
- Rosenbloom J, Castro SV, Jimenez SA. Fibrotic diseases: cellular and molecular mechanisms and novel therapies. Ann Intern Med. 2010;152:159–166. - PubMed
-
- Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmcol Rev. 2011;63:218–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

