A GAL4-driver line resource for Drosophila neurobiology
- PMID: 23063364
- PMCID: PMC3515021
- DOI: 10.1016/j.celrep.2012.09.011
A GAL4-driver line resource for Drosophila neurobiology
Abstract
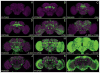
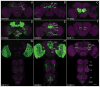
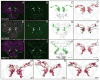
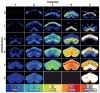
We established a collection of 7,000 transgenic lines of Drosophila melanogaster. Expression of GAL4 in each line is controlled by a different, defined fragment of genomic DNA that serves as a transcriptional enhancer. We used confocal microscopy of dissected nervous systems to determine the expression patterns driven by each fragment in the adult brain and ventral nerve cord. We present image data on 6,650 lines. Using both manual and machine-assisted annotation, we describe the expression patterns in the most useful lines. We illustrate the utility of these data for identifying novel neuronal cell types, revealing brain asymmetry, and describing the nature and extent of neuronal shape stereotypy. The GAL4 lines allow expression of exogenous genes in distinct, small subsets of the adult nervous system. The set of DNA fragments, each driving a documented expression pattern, will facilitate the generation of additional constructs for manipulating neuronal function.
Copyright © 2012 The Authors. Published by Elsevier Inc. All rights reserved.
Figures


Similar articles
-
Expanding the Drosophila toolkit for dual control of gene expression.Elife. 2024 Apr 3;12:RP94073. doi: 10.7554/eLife.94073. Elife. 2024. PMID: 38569007 Free PMC article.
-
A searchable image resource of Drosophila GAL4 driver expression patterns with single neuron resolution.Elife. 2023 Feb 23;12:e80660. doi: 10.7554/eLife.80660. Elife. 2023. PMID: 36820523 Free PMC article.
-
Molecular and cytological analysis of widely-used Gal4 driver lines for Drosophila neurobiology.BMC Genet. 2020 Oct 22;21(Suppl 1):96. doi: 10.1186/s12863-020-00895-7. BMC Genet. 2020. PMID: 33092520 Free PMC article.
-
A resource for manipulating gene expression and analyzing cis-regulatory modules in the Drosophila CNS.Cell Rep. 2012 Oct 25;2(4):1002-13. doi: 10.1016/j.celrep.2012.09.009. Epub 2012 Oct 11. Cell Rep. 2012. PMID: 23063363 Free PMC article.
-
The GAL4 System: A Versatile System for the Manipulation and Analysis of Gene Expression.Methods Mol Biol. 2016;1478:33-52. doi: 10.1007/978-1-4939-6371-3_2. Methods Mol Biol. 2016. PMID: 27730574 Review.
Cited by
-
A closed-loop optogenetic screen for neurons controlling feeding in Drosophila.G3 (Bethesda). 2021 May 7;11(5):jkab073. doi: 10.1093/g3journal/jkab073. G3 (Bethesda). 2021. PMID: 33714999 Free PMC article.
-
Collective nuclear behavior shapes bilateral nuclear symmetry for subsequent left-right asymmetric morphogenesis in Drosophila.Development. 2021 Sep 15;148(18):dev198507. doi: 10.1242/dev.198507. Epub 2021 Apr 29. Development. 2021. PMID: 34097729 Free PMC article.
-
Sleep deprivation results in diverse patterns of synaptic scaling across the Drosophila mushroom bodies.Curr Biol. 2021 Aug 9;31(15):3248-3261.e3. doi: 10.1016/j.cub.2021.05.018. Epub 2021 Jun 8. Curr Biol. 2021. PMID: 34107302 Free PMC article.
-
Dopamine promotes head direction plasticity during orienting movements.Nature. 2022 Dec;612(7939):316-322. doi: 10.1038/s41586-022-05485-4. Epub 2022 Nov 30. Nature. 2022. PMID: 36450986 Free PMC article.
-
Neuronal calcium spikes enable vector inversion in the Drosophila brain.bioRxiv [Preprint]. 2023 Nov 28:2023.11.24.568537. doi: 10.1101/2023.11.24.568537. bioRxiv. 2023. PMID: 38077032 Free PMC article. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

