Dynamics of protein and its hydration water: neutron scattering studies on fully deuterated GFP
- PMID: 23062349
- PMCID: PMC3471459
- DOI: 10.1016/j.bpj.2012.08.046
Dynamics of protein and its hydration water: neutron scattering studies on fully deuterated GFP
Abstract
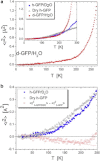
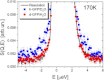
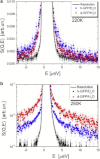
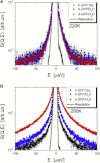
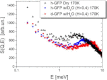
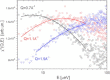
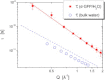
We present a detailed analysis of the picosecond-to-nanosecond motions of green fluorescent protein (GFP) and its hydration water using neutron scattering spectroscopy and hydrogen/deuterium contrast. The analysis reveals that hydration water suppresses protein motions at lower temperatures (<~ 200 K), and facilitates protein dynamics at high temperatures. Experimental data demonstrate that the hydration water is harmonic at temperatures <~ 180-190 K and is not affected by the proteins' methyl group rotations. The dynamics of the hydration water exhibits changes at ~ 180-190 K that we ascribe to the glass transition in the hydrated protein. Our results confirm significant differences in the dynamics of protein and its hydration water at high temperatures: on the picosecond-to-nanosecond timescale, the hydration water exhibits diffusive dynamics, while the protein motions are localized to <~3 Å. The diffusion of the GFP hydration water is similar to the behavior of hydration water previously observed for other proteins. Comparison with other globular proteins (e.g., lysozyme) reveals that on the timescale of 1 ns and at equivalent hydration level, GFP dynamics (mean-square displacements and quasielastic intensity) are of much smaller amplitude. Moreover, the suppression of the protein dynamics by the hydration water at low temperatures appears to be stronger in GFP than in other globular proteins. We ascribe this observation to the barrellike structure of GFP.
Copyright © 2012 Biophysical Society. Published by Elsevier Inc. All rights reserved.
Figures
Similar articles
-
Dynamics in protein powders on the nanosecond-picosecond time scale are dominated by localized motions.J Phys Chem B. 2013 Oct 3;117(39):11548-55. doi: 10.1021/jp4058884. Epub 2013 Sep 18. J Phys Chem B. 2013. PMID: 24007515
-
Coherent neutron scattering and collective dynamics in the protein, GFP.Biophys J. 2013 Nov 5;105(9):2182-7. doi: 10.1016/j.bpj.2013.09.029. Biophys J. 2013. PMID: 24209864 Free PMC article.
-
Dynamics of hydration water in deuterated purple membranes explored by neutron scattering.Eur Biophys J. 2008 Jun;37(5):619-26. doi: 10.1007/s00249-008-0285-0. Epub 2008 Feb 20. Eur Biophys J. 2008. PMID: 18286273 Free PMC article.
-
Protein dynamics investigated by neutron scattering.Photosynth Res. 2009 Nov-Dec;102(2-3):281-93. doi: 10.1007/s11120-009-9480-9. Epub 2009 Sep 11. Photosynth Res. 2009. PMID: 19763874 Review.
-
Recent results on hydrogen and hydration in biology studied by neutron macromolecular crystallography.Cell Mol Life Sci. 2006 Feb;63(3):285-300. doi: 10.1007/s00018-005-5418-3. Cell Mol Life Sci. 2006. PMID: 16389451 Free PMC article. Review.
Cited by
-
Water dynamics in MCF-7 breast cancer cells: a neutron scattering descriptive study.Sci Rep. 2019 Jun 18;9(1):8704. doi: 10.1038/s41598-019-45056-8. Sci Rep. 2019. PMID: 31213625 Free PMC article.
-
On scattered waves and lipid domains: detecting membrane rafts with X-rays and neutrons.Soft Matter. 2015 Dec 21;11(47):9055-72. doi: 10.1039/c5sm01807b. Soft Matter. 2015. PMID: 26428538 Free PMC article.
-
Correlative and integrated light and electron microscopy of in-resin GFP fluorescence, used to localise diacylglycerol in mammalian cells.Ultramicroscopy. 2014 Aug;143(100):3-14. doi: 10.1016/j.ultramic.2014.02.001. Epub 2014 Feb 22. Ultramicroscopy. 2014. PMID: 24637200 Free PMC article.
-
Experimental mapping of short-wavelength phonons in proteins.Innovation (Camb). 2021 Dec 17;3(1):100199. doi: 10.1016/j.xinn.2021.100199. eCollection 2022 Jan 25. Innovation (Camb). 2021. PMID: 35059681 Free PMC article.
-
Dynamics of a family of cyan fluorescent proteins probed by incoherent neutron scattering.J R Soc Interface. 2019 Mar 29;16(152):20180848. doi: 10.1098/rsif.2018.0848. J R Soc Interface. 2019. PMID: 30836899 Free PMC article.
References
-
- Austin R.H., Beeson K.W., Gunsalus I.C. Dynamics of ligand binding to myoglobin. Biochemistry. 1975;14:5355–5373. - PubMed
-
- Frauenfelder H., Sligar S.G., Wolynes P.G. The energy landscapes and motions of proteins. Science. 1991;254:1598–1603. - PubMed
-
- Henzler-Wildman K., Kern D. Dynamic personalities of proteins. Nature. 2007;450:964–972. - PubMed
-
- Henzler-Wildman K.A., Lei M., Kern D. A hierarchy of timescales in protein dynamics is linked to enzyme catalysis. Nature. 2007;450:913–916. - PubMed
-
- Beece D., Eisenstein L., Yue K.T. Solvent viscosity and protein dynamics. Biochemistry. 1980;19:5147–5157. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

