Comprehensive mass spectrometric mapping of the hydroxylated amino acid residues of the α1(V) collagen chain
- PMID: 23060441
- PMCID: PMC3504773
- DOI: 10.1074/jbc.M112.406850
Comprehensive mass spectrometric mapping of the hydroxylated amino acid residues of the α1(V) collagen chain
Abstract
Background: α1(V) is an extensively modified collagen chain important in disease.
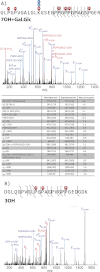
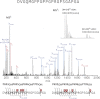
Results: Comprehensive mapping of α1(V) post-translational modifications reveals unexpectedly large numbers of X-position hydroxyprolines in Gly-X-Y amino acid triplets.
Conclusion: The unexpected abundance of X-position hydroxyprolines suggests a mechanism for differential modification of collagen properties.
Significance: Positions, numbers, and occupancy of modified sites can provide insights into α1(V) biological properties. Aberrant expression of the type V collagen α1(V) chain can underlie the connective tissue disorder classic Ehlers-Danlos syndrome, and autoimmune responses against the α1(V) chain are linked to lung transplant rejection and atherosclerosis. The α1(V) collagenous COL1 domain is thought to contain greater numbers of post-translational modifications (PTMs) than do similar domains of other fibrillar collagen chains, PTMs consisting of hydroxylated prolines and lysines, the latter of which can be glycosylated. These types of PTMs can contribute to epitopes that underlie immune responses against collagens, and the high level of PTMs may contribute to the unique biological properties of the α1(V) chain. Here we use high resolution mass spectrometry to map such PTMs in bovine placental α1(V) and human recombinant pro-α1(V) procollagen chains. Findings include the locations of those PTMs that vary and those PTMs that are invariant between these α1(V) chains from widely divergent sources. Notably, an unexpectedly large number of hydroxyproline residues were mapped to the X-positions of Gly-X-Y triplets, contrary to expectations based on previous amino acid analyses of hydrolyzed α1(V) chains from various tissues. We attribute this difference to the ability of tandem mass spectrometry coupled to nanoflow chromatographic separations to detect lower-level PTM combinations with superior sensitivity and specificity. The data are consistent with the presence of a relatively large number of 3-hydroxyproline sites with less than 100% occupancy, suggesting a previously unknown mechanism for the differential modification of α1(V) chain and type V collagen properties.
Figures


Similar articles
-
Location of 3-hydroxyproline residues in collagen types I, II, III, and V/XI implies a role in fibril supramolecular assembly.J Biol Chem. 2010 Jan 22;285(4):2580-90. doi: 10.1074/jbc.M109.068726. Epub 2009 Nov 23. J Biol Chem. 2010. PMID: 19940144 Free PMC article.
-
Characterization of the six zebrafish clade B fibrillar procollagen genes, with evidence for evolutionarily conserved alternative splicing within the pro-alpha1(V) C-propeptide.Matrix Biol. 2010 May;29(4):261-75. doi: 10.1016/j.matbio.2010.01.006. Epub 2010 Jan 25. Matrix Biol. 2010. PMID: 20102740 Free PMC article.
-
The pro-alpha3(V) collagen chain. Complete primary structure, expression domains in adult and developing tissues, and comparison to the structures and expression domains of the other types V and XI procollagen chains.J Biol Chem. 2000 Mar 24;275(12):8749-59. doi: 10.1074/jbc.275.12.8749. J Biol Chem. 2000. PMID: 10722718
-
Collagens.Cell Tissue Res. 2010 Jan;339(1):247-57. doi: 10.1007/s00441-009-0844-4. Epub 2009 Aug 20. Cell Tissue Res. 2010. PMID: 19693541 Free PMC article. Review.
-
Type V Collagen in Health, Disease, and Fibrosis.Anat Rec (Hoboken). 2016 May;299(5):613-29. doi: 10.1002/ar.23330. Epub 2016 Mar 15. Anat Rec (Hoboken). 2016. PMID: 26910848 Review.
Cited by
-
Quantitative proteomic profiling of extracellular matrix and site-specific collagen post-translational modifications in an in vitro model of lung fibrosis.Matrix Biol Plus. 2019 Apr 13;1:100005. doi: 10.1016/j.mbplus.2019.04.002. eCollection 2019 Feb. Matrix Biol Plus. 2019. PMID: 33543004 Free PMC article.
-
Impact of Bioinformatics Search Parameters for Peptides' Identification and Their Post-Translational Modifications: A Case Study of Proteolysed Gelatines from Beef, Pork, and Fish.Foods. 2023 Jun 28;12(13):2524. doi: 10.3390/foods12132524. Foods. 2023. PMID: 37444262 Free PMC article.
-
Heterogeneity in proline hydroxylation of fibrillar collagens observed by mass spectrometry.PLoS One. 2021 Aug 31;16(8):e0250544. doi: 10.1371/journal.pone.0250544. eCollection 2021. PLoS One. 2021. PMID: 34464391 Free PMC article.
-
High-resolution filtering for improved small molecule identification via GC/MS.Anal Chem. 2015 Aug 18;87(16):8328-35. doi: 10.1021/acs.analchem.5b01503. Epub 2015 Aug 7. Anal Chem. 2015. PMID: 26192401 Free PMC article.
-
Autoimmune Reactivity in Graft Injury: Player or Bystander?Curr Transplant Rep. 2015 Sep;2(3):211-221. doi: 10.1007/s40472-015-0068-3. Epub 2015 Jul 7. Curr Transplant Rep. 2015. PMID: 29057202 Free PMC article.
References
-
- Fichard A., Kleman J. P., Ruggiero F. (1995) Another look at collagen V and XI molecules. Matrix Biol 14, 515–531 - PubMed
-
- Imamura Y., Scott I. C., Greenspan D. S. (2000) The pro-α3(V) collagen chain. Complete primary structure, expression domains in adult and developing tissues, and comparison to the structures and expression domains of the other types V and XI procollagen chains. J. Biol. Chem. 275, 8749–8759 - PubMed
-
- Morris N. P., Bächinger H. P. (1987) Type XI collagen is a heterotrimer with the composition (1α, 2α, 3α) retaining non-triple-helical domains. J. Biol. Chem. 262, 11345–11350 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

