A20 inhibits LUBAC-mediated NF-κB activation by binding linear polyubiquitin chains via its zinc finger 7
- PMID: 23032186
- PMCID: PMC3463847
- DOI: 10.1038/emboj.2012.240
A20 inhibits LUBAC-mediated NF-κB activation by binding linear polyubiquitin chains via its zinc finger 7
Abstract
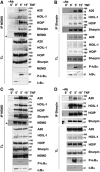
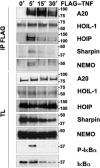
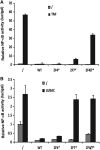
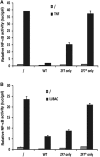
Linear polyubiquitination of proteins has recently been implicated in NF-κB signalling and is mediated by the linear ubiquitin chain assembly complex (LUBAC), consisting of HOIL-1, HOIP and Sharpin. However, the mechanisms that regulate linear ubiquitination are still unknown. Here, we show that A20 is rapidly recruited to NEMO and LUBAC upon TNF stimulation and that A20 inhibits LUBAC-induced NF-κB activation via its C-terminal zinc-finger 7 (ZF7) domain. Expression of a polypeptide corresponding to only ZF7 was sufficient to inhibit TNF-induced NF-κB activation. Both A20 and ZF7 can form a complex with NEMO and LUBAC, and are able to prevent the TNF-induced binding of NEMO to LUBAC. Finally, we show that ZF7 preferentially binds linear polyubiquitin chains in vitro, indicating A20-ZF7 as a novel linear ubiquitin-binding domain (LUBID). We thus propose a model in which A20 inhibits TNF- and LUBAC-induced NF-κB signalling by binding to linear polyubiquitin chains via its seventh zinc finger, which prevents the TNF-induced interaction between LUBAC and NEMO.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures

Similar articles
-
Specific recognition of linear polyubiquitin by A20 zinc finger 7 is involved in NF-κB regulation.EMBO J. 2012 Oct 3;31(19):3856-70. doi: 10.1038/emboj.2012.241. Epub 2012 Aug 28. EMBO J. 2012. PMID: 23032187 Free PMC article.
-
Mechanism underlying IκB kinase activation mediated by the linear ubiquitin chain assembly complex.Mol Cell Biol. 2014 Apr;34(7):1322-35. doi: 10.1128/MCB.01538-13. Epub 2014 Jan 27. Mol Cell Biol. 2014. PMID: 24469399 Free PMC article.
-
Essential role of the linear ubiquitin chain assembly complex and TAK1 kinase in A20 mutant Hodgkin lymphoma.Proc Natl Acad Sci U S A. 2020 Nov 17;117(46):28980-28991. doi: 10.1073/pnas.2014470117. Epub 2020 Nov 2. Proc Natl Acad Sci U S A. 2020. PMID: 33139544 Free PMC article.
-
Linear ubiquitination-mediated NF-κB regulation and its related disorders.J Biochem. 2013 Oct;154(4):313-23. doi: 10.1093/jb/mvt079. Epub 2013 Aug 21. J Biochem. 2013. PMID: 23969028 Review.
-
Linear ubiquitination: a novel NF-κB regulatory mechanism for inflammatory and immune responses by the LUBAC ubiquitin ligase complex.Endocr J. 2012;59(8):641-52. doi: 10.1507/endocrj.ej12-0148. Epub 2012 May 19. Endocr J. 2012. PMID: 22673407 Review.
Cited by
-
LUBAC-mediated linear ubiquitination: a crucial regulator of immune signaling.Proc Jpn Acad Ser B Phys Biol Sci. 2021;97(3):120-133. doi: 10.2183/pjab.97.007. Proc Jpn Acad Ser B Phys Biol Sci. 2021. PMID: 33692228 Free PMC article. Review.
-
Linear ubiquitination by LUBEL has a role in Drosophila heat stress response.EMBO Rep. 2016 Nov;17(11):1624-1640. doi: 10.15252/embr.201642378. Epub 2016 Oct 4. EMBO Rep. 2016. PMID: 27702987 Free PMC article.
-
Two phases of inflammatory mediator production defined by the study of IRAK2 and IRAK1 knock-in mice.J Immunol. 2013 Sep 1;191(5):2717-30. doi: 10.4049/jimmunol.1203268. Epub 2013 Aug 5. J Immunol. 2013. PMID: 23918981 Free PMC article.
-
A20 at the Crossroads of Cell Death, Inflammation, and Autoimmunity.Cold Spring Harb Perspect Biol. 2020 Jan 2;12(1):a036418. doi: 10.1101/cshperspect.a036418. Cold Spring Harb Perspect Biol. 2020. PMID: 31427375 Free PMC article. Review.
-
Distinct phylogenetic relationships and biochemical properties of Arabidopsis ovarian tumor-related deubiquitinases support their functional differentiation.Front Plant Sci. 2014 Mar 12;5:84. doi: 10.3389/fpls.2014.00084. eCollection 2014. Front Plant Sci. 2014. PMID: 24659992 Free PMC article.
References
-
- Boone DL, Turer EE, Lee EG, Ahmad RC, Wheeler MT, Tsui C, Hurley P, Chien M, Chai S, Hitotsumatsu O, McNally E, Pickart C, Ma A (2004) The ubiquitin-modifying enzyme A20 is required for termination of Toll-like receptor responses. Nat Immunol 5: 1052–1060 - PubMed
-
- Bosanac I, Wertz IE, Pan B, Yu C, Kusam S, Lam C, Phu L, Phung Q, Maurer B, Arnott D, Kirkpatrick DS, Dixit VM, Hymowitz SG (2010) Ubiquitin binding to A20 ZnF4 is required for modulation of NF-kappaB signaling. Mol Cell 40: 548–557 - PubMed
-
- Chu Y, Vahl JC, Kumar D, Heger K, Bertossi A, Wojtowicz E, Soberon V, Schenten D, Mack B, Reutelshofer M, Beyaert R, Amann K, van Loo G, Schmidt-Supprian M (2011) B cells lacking the tumor suppressor TNFAIP3/A20 display impaired differentiation and hyperactivation and cause inflammation and autoimmunity in aged mice. Blood 117: 2227–2236 - PubMed
-
- Gerlach B, Cordier SM, Schmukle AC, Emmerich CH, Rieser E, Haas TL, Webb AI, Rickard JA, Anderton H, Wong WWL, Nachbur U, Gangoda L, Warnken U, Purcell AW, Silke J, Walczak H (2011) Linear ubiquitination prevents inflammation and regulates immune signalling. Nature 471: 591–596 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

