Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis
- PMID: 23029002
- PMCID: PMC3446899
- DOI: 10.1371/journal.pone.0045427
Autophagy mediates the delivery of thrombogenic tissue factor to neutrophil extracellular traps in human sepsis
Abstract
Background: Sepsis is associated with systemic inflammatory responses and induction of coagulation system. Neutrophil extracellular traps (NETs) constitute an antimicrobial mechanism, recently implicated in thrombosis via platelet entrapment and aggregation.
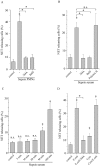
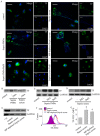
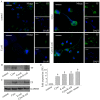
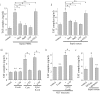
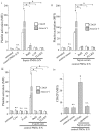
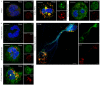
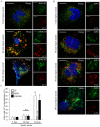
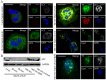
Methodology/principal findings: In this study, we demonstrate for the first time the localization of thrombogenic tissue factor (TF) in NETs released by neutrophils derived from patients with gram-negative sepsis and normal neutrophils treated with either serum from septic patients or inflammatory mediators involved in the pathogenesis of sepsis. Localization of TF in acidified autophagosomes was observed during this process, as indicated by positive LC3B and LysoTracker staining. Moreover, phosphatidylinositol 3-kinase inhibition with 3-MA or inhibition of endosomal acidification with bafilomycin A1 hindered the release of TF-bearing NETs. TF present in NETs induced thrombin generation in culture supernatants, which further resulted in protease activated receptor-1 signaling.
Conclusions/significance: This study demonstrates the involvement of autophagic machinery in the extracellular delivery of TF in NETs and the subsequent activation of coagulation cascade, providing evidence for the implication of this process in coagulopathy and inflammatory response in sepsis.
Conflict of interest statement
Figures
Similar articles
-
The emerging role of neutrophils in thrombosis-the journey of TF through NETs.Front Immunol. 2012 Dec 18;3:385. doi: 10.3389/fimmu.2012.00385. eCollection 2012. Front Immunol. 2012. PMID: 23264778 Free PMC article.
-
Tissue factor expression in neutrophil extracellular traps and neutrophil derived microparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease.Ann Rheum Dis. 2014 Oct;73(10):1854-63. doi: 10.1136/annrheumdis-2013-203430. Epub 2013 Jul 19. Ann Rheum Dis. 2014. PMID: 23873874
-
Tissue Factor-Enriched Neutrophil Extracellular Traps Promote Immunothrombosis and Disease Progression in Sepsis-Induced Lung Injury.Front Cell Infect Microbiol. 2021 Jul 14;11:677902. doi: 10.3389/fcimb.2021.677902. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34336711 Free PMC article.
-
Autophagy-driven neutrophil extracellular traps: The dawn of sepsis.Pathol Res Pract. 2022 Jun;234:153896. doi: 10.1016/j.prp.2022.153896. Epub 2022 Apr 18. Pathol Res Pract. 2022. PMID: 35462228 Review.
-
Novel Aspects of Extracellular Vesicles as Mediators of Cancer-Associated Thrombosis.Cells. 2019 Jul 13;8(7):716. doi: 10.3390/cells8070716. Cells. 2019. PMID: 31337034 Free PMC article. Review.
Cited by
-
Gradient Infiltration of Neutrophil Extracellular Traps in Colon Cancer and Evidence for Their Involvement in Tumour Growth.PLoS One. 2016 May 2;11(5):e0154484. doi: 10.1371/journal.pone.0154484. eCollection 2016. PLoS One. 2016. PMID: 27136460 Free PMC article.
-
A Review of Radiation-Induced Coagulopathy and New Findings to Support Potential Prevention Strategies and Treatments.Radiat Res. 2016 Aug;186(2):121-40. doi: 10.1667/RR14406.1. Epub 2016 Jul 26. Radiat Res. 2016. PMID: 27459701 Free PMC article.
-
Elucidating mechanisms of toxicity using phenotypic data from primary human cell systems--a chemical biology approach for thrombosis-related side effects.Int J Mol Sci. 2015 Jan 5;16(1):1008-29. doi: 10.3390/ijms16011008. Int J Mol Sci. 2015. PMID: 25569083 Free PMC article.
-
Dysregulation of neutrophil death in sepsis.Front Immunol. 2022 Aug 18;13:963955. doi: 10.3389/fimmu.2022.963955. eCollection 2022. Front Immunol. 2022. PMID: 36059483 Free PMC article. Review.
-
Scrutinizing Mechanisms of the 'Obesity Paradox in Sepsis': Obesity Is Accompanied by Diminished Formation of Neutrophil Extracellular Traps (NETs) Due to Restricted Neutrophil-Platelet Interactions.Cells. 2021 Feb 12;10(2):384. doi: 10.3390/cells10020384. Cells. 2021. PMID: 33673387 Free PMC article.
References
-
- Mitroulis I, Kambas K, Anyfanti P, Doumas M, Ritis K (2011a) The multivalent activity of the tissue factor-thrombin pathway in thrombotic and non-thrombotic disorders as a target for therapeutic intervention. Expert Opin Ther Targets 15: 75–89. - PubMed
-
- Aras O, Shet A, Bach RR, Hysjulien JL, Slungaard A, et al. (2004) Induction of microparticle- and cell-associated intravascular tissue factor in human endotoxemia. Blood 103: 4545–4553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

