Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype
- PMID: 23027612
- PMCID: PMC3491837
- DOI: 10.1002/emmm.201201374
Interleukin-13 protects from atherosclerosis and modulates plaque composition by skewing the macrophage phenotype
Abstract
Atherosclerotic lesions are characterized by the accumulation of oxidized LDL (OxLDL) and the infiltration of macrophages and T cells. Cytokine expression in the microenvironment of evolving lesions can profoundly contribute to plaque development. While the pro-atherogenic effect of T helper (Th) 1 cytokines, such as IFN-γ, is well established, the role of Th2 cytokines is less clear. Therefore, we characterized the role of the Th2 cytokine interleukin (IL)-13 in murine atherosclerosis. Here, we report that IL-13 administration favourably modulated the morphology of already established atherosclerotic lesions by increasing lesional collagen content and reducing vascular cell adhesion molecule-1 (VCAM-1)-dependent monocyte recruitment, resulting in decreased plaque macrophage content. This was accompanied by the induction of alternatively activated (M2) macrophages, which exhibited increased clearance of OxLDL compared to IFN-γ-activated (M1) macrophages in vitro. Importantly, deficiency of IL-13 results in accelerated atherosclerosis in LDLR(-/-) mice without affecting plasma cholesterol levels. Thus, IL-13 protects from atherosclerosis and promotes a favourable plaque morphology, in part through the induction of alternatively activated macrophages.
Copyrights © 2012 The Authors. Published by John Wiley and Sons, Ltd on behalf of EMBO.
Figures
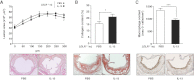
LDLR−/− mice were fed an atherogenic diet for 16 weeks and received biweekly intraperitoneal injections with PBS (n = 11) or IL-13 (n = 13) during the last 5 weeks. Equal extent of atherosclerotic lesion size in cross-sections of the aortic origin in injected LDLR−/− mice. Values represent µm2/section throughout the entire aortic origin (300 µm). Original magnification 50×.
Increased collagen content in lesions of LDLR−/− mice injected with IL-13. Sections were stained with Sirius Red for the presence of collagen, and values represent the percentages of Sirius Red+ area/total lesion area. *p = 0.035. Original magnification 100×.
Decreased macrophage content in lesions of LDLR−/− mice injected with IL-13. Sections were stained with the macrophage-specific anti-mac-3 antibody and values represent the numbers of mac-3+ cells/mm2 of total lesion area. ***p = 0.0007. Original magnification 100×. All data are mean ± SEM values of all mice of each group. Images show representative examples of the respective stainings.

Diagram of experimental design.
Similar content of fluorescent beads per plaque area in the aortic root of PBS and IL-13 administration, respectively, compared to baseline.
Decreased macrophage content in lesions of ApoE−/− mice injected with IL-13. Sections were stained with the macrophage-specific anti-mac-2 antibody and values represent the percentages of mac-2+ cells/total DAPI+ cells. ***p = 0.0001. All data are shown as mean ± SEM values of all mice of each group.

Cx3cr1gfp/wt ApoE−/− mice were fed an atherogenic diet for 6 weeks and received biweekly intraperitoneal injections with PBS (n = 8) or IL-13 (n = 8) during the last 2 weeks. At the time of sacrifice, PE-conjugated anti-GR1 antibodies were injected intravenously and the number of monocytes (GFP+ cells) and neutrophils (PE+ cells) adhering to the carotid bifurcation was assessed by intravital microscopy. Values represent the numbers of cells/optical field. *p = 0.0185, Mann–Whitney test.
ApoE−/− mice were fed an atherogenic diet for 6 weeks and received biweekly intraperitoneal injections with PBS (n = 8) or IL-13 (n = 8) during the last 2 weeks. At time of sacrifice, fluorescent anti-VCAM-1 beads were injected intravenously and the number of beads adhering to the carotid bifurcation was assessed by intravital microscopy. Values represent the numbers of beads/optical field. ***p = 0.0001. Representative microscopy images are shown. Bar: 100 µm. All data are shown as mean ± SEM values of all mice of each group.

LDLR−/− mice were fed an atherogenic diet for 16 weeks and received biweekly intraperitoneal injections with PBS (n = 11) or IL-13 (n = 13) during the last 5 weeks. Increased frequencies of M2 macrophages and decreased frequencies of M1 macrophages in the peritoneal cavities of LDLR−/− mice injected with IL-13. Peritoneal cells were stained with antibodies against CD80 (M1) and CD206 (M2) and identified by flow cytometry. CD80/CD206 double-positive cells were classified as M1/M2 macrophages. Values represent the percentages of individual macrophage subtypes/total macrophages. *p = 0.04.
Decreased numbers of M1 macrophages in lesions of LDLR−/− mice injected with IL-13. Sections were stained with an antibody against iNOS, which is specifically expressed by M1 macrophages and values represent the numbers of iNOS+ cells/mm2 of total lesion area. *p = 0.012.
Increased numbers of M2 macrophages in lesions of LDLR−/− mice injected with IL-13. Sections were stained with an antibody against Ym-1, which is specifically expressed by M2 macrophages. Values represent the numbers of Ym-1+ cells/mm2 of total lesion area. *p = 0.024.
Increased ratio of M2:M1 macrophages in lesions of LDLR−/− mice injected with IL-13. ***p = 0.0001. All data are shown as mean ± SEM values of all mice of each group. Images show representative examples of the respective stainings. Original magnification: 400×.
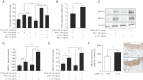
A. Thioglycollate-elicited macrophages were stimulated with IFN-γ or IL-13 into classically (M1) or alternatively (M2) activated macrophages, respectively, and then incubated with CuOx-LDL for 24 h to generate foam cells. Increased cellular cholesterol levels in M2-derived foam cells are reduced in the presence of HDL. M1 and M2 macrophages were incubated with CuOx-LDL in the absence or presence of HDL 10 µg/ml. Lipids were extracted from cell lysates and total cholesterol and protein were measured. Data are shown as mean ± SEM values of two independent experiments performed in quadruplicates and represent mg cholesterol/mg protein. *p = 0.04, ***p = 0.0001.
B. Increased HDL-dependent cholesterol efflux by M2-derived foam cells. M1 and M2 macrophages were incubated with CuOx-LDL plus 1 µM of [3H]-cholesterol and HDL-dependent efflux was assayed as described in Materials and Methods Section. Data represent percentages of HDL-dependent efflux/total efflux. *p = 0.010, t-test.
C. Increased ABCA1 and ABCG1 expression in M2-derived foam cells. Shown is a representative Western blot for the presence of ABCA1, ABCG1 and β-actin in lysates of cells that were treated as indicated.
D,E. The quantification of the band intensity of ABCA1 (D) and ABCG1 (E) related to β-actin. *p = 0.04, **p = 0.0075, ***p = 0.0001. All data in (B–E) are shown as mean ± SEM values of three independent experiments performed in triplicates.
F. Increased ABCA1 expression in lesions of LDLR−/− mice injected with IL-13. LDLR−/− mice were fed an atherogenic diet for 16 weeks and received biweekly intraperitoneal injections with PBS (n = 11) or IL-13 (n = 13) during the last 5 weeks. Sections were stained with an antibody against ABCA1 and values represent the numbers of ABCA1+ cells/mm2 of total lesion area, *p = 0.031. Data are shown as mean ± SEM values of all mice of each group. Images show representative ABCA1 staining. Original magnification 400×.
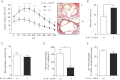
LDLR−/− mice were reconstituted with bone marrow from either IL-13+/+ mice (n = 12) or IL-13−/− mice (n = 14) and fed an atherogenic diet for 16 weeks. Increased extent of atherosclerotic lesion size in cross-sections of the aortic origin in mice reconstituted with IL-13−/− bone marrow. Values represent µm2/section throughout the entire aortic origin, (400 µm). **p = 0.0023. Images show representative H&E stains. Original magnification 50×.
Increased necrotic core area in lesions of recipients of IL-13−/− bone marrow. Values represent percentages of necrotic core area/total lesion area. *p = 0.016.
Lesional macrophage content between recipients of IL-13−/− or IL-13+/+ bone marrow. Sections were stained with the macrophage-specific anti-mac-3 antibody and values represent the percentages of mac-3+ area/cellular lesion area.
Decreased lesional M2 macrophage content in lesions of recipients of IL-13−/− bone marrow. Sections were stained with an antibody against Ym-1, which is specifically expressed by M2 macrophages and values represent number of Ym-1+ cells/mm2 of cellular lesion area. **p = 0.0043, Mann–Whitney test.
Relative lesional M1 macrophage content between recipients of IL-13−/− or IL-13+/+ bone marrow. Sections were stained with an antibody against iNOS, which is specifically expressed by M1 macrophages and values represent the percentages of iNOS+ area/cellular lesion area. All data are shown as mean ± SEM values of all mice of each group.
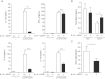
LDLR−/− mice were reconstituted with bone marrow from either IL-13+/+ mice (n = 12) or IL-13−/− mice (n = 14) and fed an atherogenic diet for 16 weeks. At time of sacrifice, spleens and blood were collected from all mice. Recipients of IL-13-deficient bone marrow show a decreased production of Th2 cytokines (IL-13, IL-4 and IL-10) but not IFN-γ by splenocytes stimulated with anti-CD3/CD28 in vitro. Data are presented as ng/ml cytokine of splenocyte cultures ***p = 0.0001.
Increased levels of total IgG2c antibodies in sera of IL-13-deficient LDLR−/− mice. Data are presented as mg/ml of indicated serum IgG isotypes. *p = 0.029.
Decreased ratio of IgG1:IgG2c antibodies in IL-13-deficient LDLR−/− mice. *p = 0.04. All data are shown as mean ± SEM values of all mice of each group.
Similar articles
-
Prevention of oxLDL uptake leads to decreased atherosclerosis in hematopoietic NPC1-deficient Ldlr-/- mice.Atherosclerosis. 2016 Dec;255:59-65. doi: 10.1016/j.atherosclerosis.2016.10.038. Epub 2016 Oct 20. Atherosclerosis. 2016. PMID: 27816810
-
IgE Contributes to Atherosclerosis and Obesity by Affecting Macrophage Polarization, Macrophage Protein Network, and Foam Cell Formation.Arterioscler Thromb Vasc Biol. 2020 Mar;40(3):597-610. doi: 10.1161/ATVBAHA.119.313744. Epub 2020 Jan 30. Arterioscler Thromb Vasc Biol. 2020. PMID: 31996021 Free PMC article.
-
Hematopoietic arginase 1 deficiency results in decreased leukocytosis and increased foam cell formation but does not affect atherosclerosis.Atherosclerosis. 2017 Jan;256:35-46. doi: 10.1016/j.atherosclerosis.2016.11.018. Epub 2016 Nov 16. Atherosclerosis. 2017. PMID: 27998825
-
Macrophage phenotypes in atherosclerosis.Immunol Rev. 2014 Nov;262(1):153-66. doi: 10.1111/imr.12218. Immunol Rev. 2014. PMID: 25319333 Review.
-
The phenomenon of atherosclerosis reversal and regression: Lessons from animal models.Exp Mol Pathol. 2017 Feb;102(1):138-145. doi: 10.1016/j.yexmp.2017.01.013. Epub 2017 Jan 17. Exp Mol Pathol. 2017. PMID: 28108216 Review.
Cited by
-
ApoB-Specific CD4+ T Cells in Mouse and Human Atherosclerosis.Cells. 2021 Feb 19;10(2):446. doi: 10.3390/cells10020446. Cells. 2021. PMID: 33669769 Free PMC article. Review.
-
Deletion of calponin 2 in macrophages alters cytoskeleton-based functions and attenuates the development of atherosclerosis.J Mol Cell Cardiol. 2016 Oct;99:87-99. doi: 10.1016/j.yjmcc.2016.08.019. Epub 2016 Aug 26. J Mol Cell Cardiol. 2016. PMID: 27575021 Free PMC article.
-
IL-13 promotes functional recovery after myocardial infarction via direct signaling to macrophages.JCI Insight. 2024 Jan 23;9(2):e172702. doi: 10.1172/jci.insight.172702. JCI Insight. 2024. PMID: 38051583 Free PMC article.
-
Macrophage Trafficking, Inflammatory Resolution, and Genomics in Atherosclerosis: JACC Macrophage in CVD Series (Part 2).J Am Coll Cardiol. 2018 Oct 30;72(18):2181-2197. doi: 10.1016/j.jacc.2018.08.2147. J Am Coll Cardiol. 2018. PMID: 30360827 Free PMC article. Review.
-
Macrophage deficiency of Akt2 reduces atherosclerosis in Ldlr null mice.J Lipid Res. 2014 Nov;55(11):2296-308. doi: 10.1194/jlr.M050633. Epub 2014 Sep 19. J Lipid Res. 2014. PMID: 25240046 Free PMC article.
References
-
- Ait-Oufella H, Taleb S, Mallat Z, Tedgui A. Recent advances on the role of cytokines in atherosclerosis. Arterioscler Thromb Vasc Biol. 2011;31:969–979. - PubMed
-
- Bancroft AJ, McKenzie AN, Grencis RK. A critical role for IL-13 in resistance to intestinal nematode infection. J Immunol. 1998;160:3453–3461. - PubMed
-
- Barlow JL, McKenzie AN. Nuocytes: expanding the innate cell repertoire in type-2 immunity. J Leukoc Biol. 2011;90:867–874. - PubMed
-
- Berry A, Balard P, Coste A, Olagnier D, Lagane C, Authier H, Benoit-Vical F, Lepert JC, Séguéla JP, Magnaval JF, et al. IL-13 induces expression of CD36 in human monocytes through PPArg activation. Eur J Immunol. 2007;37:1642–1652. - PubMed
-
- Binder CJ, Chang MK, Shaw PX, Miller YI, Hartvigsen K, Dewan A, Witztum JL. Innate and acquired immunity in atherogenesis. Nat Med. 2002;8:1218–1226. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

