What a difference an X or Y makes: sex chromosomes, gene dose, and epigenetics in sexual differentiation
- PMID: 23027446
- PMCID: PMC4150872
- DOI: 10.1007/978-3-642-30726-3_4
What a difference an X or Y makes: sex chromosomes, gene dose, and epigenetics in sexual differentiation
Abstract
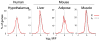
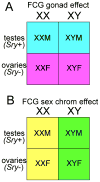
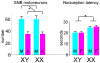
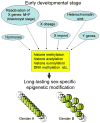
A modern general theory of sex determination and sexual differentiation identifies the factors that cause sexual bias in gene networks, leading to sex differences in physiology and disease. The primary sex-biasing factors are those encoded on the sex chromosomes that are inherently different in the male and female zygotes. These factors, and downstream factors such as gonadal hormones, act directly on tissues to produce sex differences and antagonize each other to reduce sex differences. Recent studies of mouse models such as the four core genotypes have begun to distinguish between the direct effects of sex chromosome complement (XX vs. XY) and hormonal effects. Several lines of evidence implicate epigenetic processes in the control of sex differences, although a great deal of information is needed about sex differences in the epigenome.
Figures

Similar articles
-
Genetics and Epigenetics of the X and Y Chromosomes in the Sexual Differentiation of the Brain.Int J Mol Sci. 2022 Oct 14;23(20):12288. doi: 10.3390/ijms232012288. Int J Mol Sci. 2022. PMID: 36293143 Free PMC article. Review.
-
A general theory of sexual differentiation.J Neurosci Res. 2017 Jan 2;95(1-2):291-300. doi: 10.1002/jnr.23884. J Neurosci Res. 2017. PMID: 27870435 Free PMC article. Review.
-
Rethinking sex determination of non-gonadal tissues.Curr Top Dev Biol. 2019;134:289-315. doi: 10.1016/bs.ctdb.2019.01.003. Epub 2019 Feb 12. Curr Top Dev Biol. 2019. PMID: 30999979 Free PMC article. Review.
-
Increased high-density lipoprotein cholesterol levels in mice with XX versus XY sex chromosomes.Arterioscler Thromb Vasc Biol. 2015 Aug;35(8):1778-86. doi: 10.1161/ATVBAHA.115.305460. Epub 2015 Jun 25. Arterioscler Thromb Vasc Biol. 2015. PMID: 26112012 Free PMC article.
-
Conceptual frameworks and mouse models for studying sex differences in physiology and disease: why compensation changes the game.Exp Neurol. 2014 Sep;259:2-9. doi: 10.1016/j.expneurol.2014.01.021. Epub 2014 Feb 7. Exp Neurol. 2014. PMID: 24509348 Free PMC article. Review.
Cited by
-
A P2RY12 deficiency results in sex-specific cellular perturbations and sexually dimorphic behavioral anomalies.J Neuroinflammation. 2024 Apr 15;21(1):95. doi: 10.1186/s12974-024-03079-7. J Neuroinflammation. 2024. PMID: 38622726 Free PMC article.
-
XX sex chromosome complement modulates immune responses to heat-killed Streptococcus pneumoniae immunization in a microbiome-dependent manner.Biol Sex Differ. 2024 Mar 14;15(1):21. doi: 10.1186/s13293-024-00597-0. Biol Sex Differ. 2024. PMID: 38486287 Free PMC article.
-
XX sex chromosome complement modulates immune responses to heat-killed Streptococcus pneumoniae immunization in a microbiome-dependent manner.Res Sq [Preprint]. 2023 Nov 2:rs.3.rs-3429829. doi: 10.21203/rs.3.rs-3429829/v1. Res Sq. 2023. Update in: Biol Sex Differ. 2024 Mar 14;15(1):21. doi: 10.1186/s13293-024-00597-0. PMID: 37961596 Free PMC article. Updated. Preprint.
-
Molecular basis of sex differences in cancer: Perspective from Asia.iScience. 2023 Jun 12;26(7):107101. doi: 10.1016/j.isci.2023.107101. eCollection 2023 Jul 21. iScience. 2023. PMID: 37404373 Free PMC article. Review.
-
The Male-Biased Expression of miR-2954 Is Involved in the Male Pathway of Chicken Sex Differentiation.Cells. 2022 Dec 20;12(1):4. doi: 10.3390/cells12010004. Cells. 2022. PMID: 36611798 Free PMC article.
References
-
- Arnold AP. Genetically triggered sexual differentiation of brain and behavior. Horm Behav. 1996;30:495–505. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

