Beyond cell adhesion: the role of armadillo proteins in the heart
- PMID: 23022961
- PMCID: PMC3508382
- DOI: 10.1016/j.cellsig.2012.09.025
Beyond cell adhesion: the role of armadillo proteins in the heart
Abstract
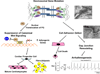
Plakoglobin (PG, γ-Catenin, JUP), a member of the armadillo protein family and close homolog of β-catenin, functions to link cell surface cadherin molecules with the cytoskeleton. PG is the only junctional component found in both desmosomes and adherens junctions and thus plays a critical role in the regulation of cell-cell adhesion. Similar to β-catenin, PG is able to interact with components of the Wnt signaling pathway and directly affect gene expression by binding with LEF/TCF transcription factors. In addition, it has been proposed that PG functions primarily as a competitive inhibitor of β-catenin transcriptional activity by sequestering LEF/TCF. Compared to β-catenin, the contribution of PG as a transcriptional regulator in either physiological or pathological conditions is poorly understood. There is increasing clinical interest in PG as both a structural protein as well as a signaling molecule as mutations have been identified in the human PG gene that cause Arrhythmogenic Right Ventricular Cardiomyopathy (ARVC) and cutaneous syndromes. This review will discuss the connection between altered cell adhesion and gene expression and its contribution to disease pathogenesis.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures

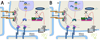

Similar articles
-
Cardiac tissue-restricted deletion of plakoglobin results in progressive cardiomyopathy and activation of {beta}-catenin signaling.Mol Cell Biol. 2011 Mar;31(6):1134-44. doi: 10.1128/MCB.01025-10. Epub 2011 Jan 18. Mol Cell Biol. 2011. PMID: 21245375 Free PMC article.
-
Analysis of a Jup hypomorphic allele reveals a critical threshold for postnatal viability.Genesis. 2012 Oct;50(10):717-27. doi: 10.1002/dvg.22034. Epub 2012 May 14. Genesis. 2012. PMID: 22522917 Free PMC article.
-
Restrictive loss of plakoglobin in cardiomyocytes leads to arrhythmogenic cardiomyopathy.Hum Mol Genet. 2011 Dec 1;20(23):4582-96. doi: 10.1093/hmg/ddr392. Epub 2011 Aug 31. Hum Mol Genet. 2011. PMID: 21880664 Free PMC article.
-
Beyond cell-cell adhesion: Plakoglobin and the regulation of tumorigenesis and metastasis.Oncotarget. 2017 May 9;8(19):32270-32291. doi: 10.18632/oncotarget.15650. Oncotarget. 2017. PMID: 28416759 Free PMC article. Review.
-
The Yin-Yang of TCF/beta-catenin signaling.Adv Cancer Res. 2000;77:1-24. doi: 10.1016/s0065-230x(08)60783-6. Adv Cancer Res. 2000. PMID: 10549354 Review.
Cited by
-
The hippo pathway is activated and is a causal mechanism for adipogenesis in arrhythmogenic cardiomyopathy.Circ Res. 2014 Jan 31;114(3):454-68. doi: 10.1161/CIRCRESAHA.114.302810. Epub 2013 Nov 25. Circ Res. 2014. PMID: 24276085 Free PMC article.
-
Kir2.1 Interactome Mapping Uncovers PKP4 as a Modulator of the Kir2.1-Regulated Inward Rectifier Potassium Currents.Mol Cell Proteomics. 2020 Sep;19(9):1436-1449. doi: 10.1074/mcp.RA120.002071. Epub 2020 Jun 15. Mol Cell Proteomics. 2020. PMID: 32541000 Free PMC article.
-
Plakoglobin as a regulator of desmocollin gene expression.J Invest Dermatol. 2013 Dec;133(12):2732-2740. doi: 10.1038/jid.2013.220. Epub 2013 May 7. J Invest Dermatol. 2013. PMID: 23652796 Free PMC article.
-
Desmosomes: Essential contributors to an integrated intercellular junction network.F1000Res. 2019 Dec 30;8:F1000 Faculty Rev-2150. doi: 10.12688/f1000research.20942.1. eCollection 2019. F1000Res. 2019. PMID: 31942240 Free PMC article. Review.
-
Understanding the molecular basis of cardiomyopathy.Am J Physiol Heart Circ Physiol. 2022 Feb 1;322(2):H181-H233. doi: 10.1152/ajpheart.00562.2021. Epub 2021 Nov 19. Am J Physiol Heart Circ Physiol. 2022. PMID: 34797172 Free PMC article. Review.
References
-
- Cowin P, Kapprell HP, Franke WW, Tamkun J, Hynes RO. Cell. 1986;46(7):1063–1073. - PubMed
-
- Peifer M, Wieschaus E. Cell. 1990;63(6):1167–1176. - PubMed
-
- McCrea PD, Turck CW, Gumbiner B. Science. 1991;254(5036):1359–1361. - PubMed
-
- Butz S, Stappert J, Weissig H, Kemler R. Science. 1992;257(5073):1142–1144. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

