The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy
- PMID: 23011132
- PMCID: PMC3657998
- DOI: 10.1038/ncomms2090
The miRNA-212/132 family regulates both cardiac hypertrophy and cardiomyocyte autophagy
Abstract
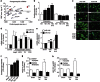
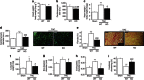
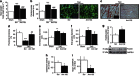
Pathological growth of cardiomyocytes (hypertrophy) is a major determinant for the development of heart failure, one of the leading medical causes of mortality worldwide. Here we show that the microRNA (miRNA)-212/132 family regulates cardiac hypertrophy and autophagy in cardiomyocytes. Hypertrophic stimuli upregulate cardiomyocyte expression of miR-212 and miR-132, which are both necessary and sufficient to drive the hypertrophic growth of cardiomyocytes. MiR-212/132 null mice are protected from pressure-overload-induced heart failure, whereas cardiomyocyte-specific overexpression of the miR-212/132 family leads to pathological cardiac hypertrophy, heart failure and death in mice. Both miR-212 and miR-132 directly target the anti-hypertrophic and pro-autophagic FoxO3 transcription factor and overexpression of these miRNAs leads to hyperactivation of pro-hypertrophic calcineurin/NFAT signalling and an impaired autophagic response upon starvation. Pharmacological inhibition of miR-132 by antagomir injection rescues cardiac hypertrophy and heart failure in mice, offering a possible therapeutic approach for cardiac failure.
Conflict of interest statement
A.U., S.K.G., K.C. and T.T. are co-inventors on a patent entitled 'Inhibition of microRNA-212/132 as a therapeutical approach for the treatment and prevention of hypertrophy and autophagy associated heart diseases.' The remaining authors declare no competing financial interests.
Figures




Similar articles
-
Reciprocal repression between microRNA-133 and calcineurin regulates cardiac hypertrophy: a novel mechanism for progressive cardiac hypertrophy.Hypertension. 2010 Apr;55(4):946-52. doi: 10.1161/HYPERTENSIONAHA.109.139519. Epub 2010 Feb 22. Hypertension. 2010. PMID: 20177001
-
Loss of MicroRNA-155 protects the heart from pathological cardiac hypertrophy.Circ Res. 2014 May 9;114(10):1585-95. doi: 10.1161/CIRCRESAHA.114.303784. Epub 2014 Mar 21. Circ Res. 2014. PMID: 24657879 Free PMC article.
-
MicroRNA-122 promotes cardiomyocyte hypertrophy via targeting FoxO3.Biochem Biophys Res Commun. 2019 Nov 19;519(4):682-688. doi: 10.1016/j.bbrc.2019.09.035. Epub 2019 Sep 19. Biochem Biophys Res Commun. 2019. PMID: 31543343
-
MicroRNA regulation and role in stem cell maintenance, cardiac differentiation and hypertrophy.Curr Mol Med. 2013 Jun;13(5):757-64. doi: 10.2174/1566524011313050007. Curr Mol Med. 2013. PMID: 23642057 Free PMC article. Review.
-
The function of miRNA in cardiac hypertrophy.Cell Mol Life Sci. 2012 Nov;69(21):3561-70. doi: 10.1007/s00018-012-1126-y. Epub 2012 Aug 25. Cell Mol Life Sci. 2012. PMID: 22926414 Free PMC article. Review.
Cited by
-
Autophagy in striated muscle diseases.Front Cardiovasc Med. 2022 Oct 13;9:1000067. doi: 10.3389/fcvm.2022.1000067. eCollection 2022. Front Cardiovasc Med. 2022. PMID: 36312227 Free PMC article. Review.
-
Current RNA strategies in treating cardiovascular diseases.Mol Ther. 2024 Mar 6;32(3):580-608. doi: 10.1016/j.ymthe.2024.01.028. Epub 2024 Jan 29. Mol Ther. 2024. PMID: 38291757 Review.
-
Microarray analysis reveals altered circulating microRNA expression in mice infected with Coxsackievirus B3.Exp Ther Med. 2016 Oct;12(4):2220-2226. doi: 10.3892/etm.2016.3607. Epub 2016 Aug 22. Exp Ther Med. 2016. PMID: 27698715 Free PMC article.
-
The study of the mechanism of non-coding RNA regulation of programmed cell death in diabetic cardiomyopathy.Mol Cell Biochem. 2024 Jul;479(7):1673-1696. doi: 10.1007/s11010-023-04909-7. Epub 2024 Jan 8. Mol Cell Biochem. 2024. PMID: 38189880 Review.
-
Hypoxia-Induced MicroRNA-212/132 Alter Blood-Brain Barrier Integrity Through Inhibition of Tight Junction-Associated Proteins in Human and Mouse Brain Microvascular Endothelial Cells.Transl Stroke Res. 2019 Dec;10(6):672-683. doi: 10.1007/s12975-018-0683-2. Epub 2019 Jan 8. Transl Stroke Res. 2019. PMID: 30617994 Free PMC article.
References
-
- Hill J. A. & Olson E. N. Cardiac plasticity. N. Engl. J. Med. 358, 1370–1380 (2008). - PubMed
-
- Barry S. P. & Townsend P. A. What causes a broken heart-molecular insights into heart failure. Int. Rev. Cell Mol. Biol. 284, 113–179 (2010). - PubMed
-
- Glas D. J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 346, 267–278 (2010). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

