A noncoding RNA produced by arthropod-borne flaviviruses inhibits the cellular exoribonuclease XRN1 and alters host mRNA stability
- PMID: 23006624
- PMCID: PMC3479393
- DOI: 10.1261/rna.034330.112
A noncoding RNA produced by arthropod-borne flaviviruses inhibits the cellular exoribonuclease XRN1 and alters host mRNA stability
Abstract
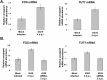
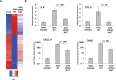
All arthropod-borne flaviviruses generate a short noncoding RNA (sfRNA) from the viral 3' untranslated region during infection due to stalling of the cellular 5'-to-3' exonuclease XRN1. We show here that formation of sfRNA also inhibits XRN1 activity. Cells infected with Dengue or Kunjin viruses accumulate uncapped mRNAs, decay intermediates normally targeted by XRN1. XRN1 repression also resulted in the increased overall stability of cellular mRNAs in flavivirus-infected cells. Importantly, a mutant Kunjin virus that cannot form sfRNA but replicates to normal levels failed to affect host mRNA stability or XRN1 activity. Expression of sfRNA in the absence of viral infection demonstrated that sfRNA formation was directly responsible for the stabilization of cellular mRNAs. Finally, numerous cellular mRNAs were differentially expressed in an sfRNA-dependent fashion in a Kunjin virus infection. We conclude that flaviviruses incapacitate XRN1 during infection and dysregulate host mRNA stability as a result of sfRNA formation.
Figures







Similar articles
-
Zika virus noncoding sfRNAs sequester multiple host-derived RNA-binding proteins and modulate mRNA decay and splicing during infection.J Biol Chem. 2019 Nov 1;294(44):16282-16296. doi: 10.1074/jbc.RA119.009129. Epub 2019 Sep 13. J Biol Chem. 2019. PMID: 31519749 Free PMC article.
-
Noncoding Subgenomic Flavivirus RNA Is Processed by the Mosquito RNA Interference Machinery and Determines West Nile Virus Transmission by Culex pipiens Mosquitoes.J Virol. 2016 Oct 28;90(22):10145-10159. doi: 10.1128/JVI.00930-16. Print 2016 Nov 15. J Virol. 2016. PMID: 27581979 Free PMC article.
-
Functional non-coding RNAs derived from the flavivirus 3' untranslated region.Virus Res. 2015 Aug 3;206:53-61. doi: 10.1016/j.virusres.2015.01.026. Epub 2015 Feb 7. Virus Res. 2015. PMID: 25660582 Review.
-
RNA structures that resist degradation by Xrn1 produce a pathogenic Dengue virus RNA.Elife. 2014 Apr 1;3:e01892. doi: 10.7554/eLife.01892. Elife. 2014. PMID: 24692447 Free PMC article.
-
New hypotheses derived from the structure of a flaviviral Xrn1-resistant RNA: Conservation, folding, and host adaptation.RNA Biol. 2015;12(11):1169-77. doi: 10.1080/15476286.2015.1094599. Epub 2015 Sep 23. RNA Biol. 2015. PMID: 26399159 Free PMC article. Review.
Cited by
-
RNase L activation in the cytoplasm induces aberrant processing of mRNAs in the nucleus.PLoS Pathog. 2022 Nov 1;18(11):e1010930. doi: 10.1371/journal.ppat.1010930. eCollection 2022 Nov. PLoS Pathog. 2022. PMID: 36318584 Free PMC article.
-
TREX tetramer disruption alters RNA processing necessary for corticogenesis in THOC6 Intellectual Disability Syndrome.Nat Commun. 2024 Feb 22;15(1):1640. doi: 10.1038/s41467-024-45948-y. Nat Commun. 2024. PMID: 38388531 Free PMC article.
-
A Tale of Two RNAs during Viral Infection: How Viruses Antagonize mRNAs and Small Non-Coding RNAs in The Host Cell.Viruses. 2016 Jun 2;8(6):154. doi: 10.3390/v8060154. Viruses. 2016. PMID: 27271653 Free PMC article. Review.
-
Evaluation in Swine of a Recombinant African Swine Fever Virus Lacking the MGF-360-1L Gene.Viruses. 2020 Oct 20;12(10):1193. doi: 10.3390/v12101193. Viruses. 2020. PMID: 33092258 Free PMC article.
-
Interplay between viruses and host mRNA degradation.Biochim Biophys Acta. 2013 Jun-Jul;1829(6-7):732-41. doi: 10.1016/j.bbagrm.2012.12.003. Epub 2012 Dec 26. Biochim Biophys Acta. 2013. PMID: 23274304 Free PMC article. Review.
References
-
- Astuti D, Morris MR, Cooper WN, Staals RH, Wake NC, Fews GA, Gill H, Gentle D, Shuib S, Ricketts CJ, et al. 2012. Germline mutations in DIS3L2 cause the Perlman syndrome of overgrowth and Wilms tumor susceptibility. Nat Genet 44: 277–284 - PubMed
-
- Bregman A, Avraham-Kelbert M, Barkai O, Duek L, Guterman A, Choder M 2011. Promoter elements regulate cytoplasmic mRNA decay. Cell 147: 1473–1483 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
