Dll1+ secretory progenitor cells revert to stem cells upon crypt damage
- PMID: 23000963
- PMCID: PMC3789123
- DOI: 10.1038/ncb2581
Dll1+ secretory progenitor cells revert to stem cells upon crypt damage
Abstract
Lgr5+ intestinal stem cells generate enterocytes and secretory cells. Secretory lineage commitment requires Notch silencing. The Notch ligand Dll1 is expressed by a subset of immediate stem cell daughters. Lineage tracing in Dll1(GFP-ires-CreERT2) knock-in mice reveals that single Dll1(high) cells generate small, short-lived clones containing all four secretory cell types. Lineage specification thus occurs in immediate stem cell daughters through Notch lateral inhibition. Cultured Dll1(high) cells form long-lived organoids (mini-guts) on brief Wnt3A exposure. When Dll1(high) cells are genetically marked before tissue damage, stem cell tracing events occur. Thus, secretory progenitors exhibit plasticity by regaining stemness on damage.
Figures
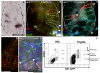


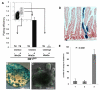
Similar articles
-
BHLHA15-Positive Secretory Precursor Cells Can Give Rise to Tumors in Intestine and Colon in Mice.Gastroenterology. 2019 Mar;156(4):1066-1081.e16. doi: 10.1053/j.gastro.2018.11.024. Epub 2018 Nov 15. Gastroenterology. 2019. PMID: 30448068 Free PMC article.
-
Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells.Gastroenterology. 2011 Apr;140(4):1230-1240.e1-7. doi: 10.1053/j.gastro.2011.01.005. Epub 2011 Jan 14. Gastroenterology. 2011. PMID: 21238454 Free PMC article.
-
Radical and lunatic fringes modulate notch ligands to support mammalian intestinal homeostasis.Elife. 2018 Apr 9;7:e35710. doi: 10.7554/eLife.35710. Elife. 2018. PMID: 29629872 Free PMC article.
-
Oscillatory Control of Notch Signaling in Development.Adv Exp Med Biol. 2018;1066:265-277. doi: 10.1007/978-3-319-89512-3_13. Adv Exp Med Biol. 2018. PMID: 30030831 Review.
-
An oscillatory network controlling self-renewal of skeletal muscle stem cells.Exp Cell Res. 2021 Dec 15;409(2):112933. doi: 10.1016/j.yexcr.2021.112933. Epub 2021 Nov 15. Exp Cell Res. 2021. PMID: 34793773 Review.
Cited by
-
MicroRNA-222 Regulates Melanoma Plasticity.J Clin Med. 2020 Aug 8;9(8):2573. doi: 10.3390/jcm9082573. J Clin Med. 2020. PMID: 32784455 Free PMC article.
-
Frizzled7 functions as a Wnt receptor in intestinal epithelial Lgr5(+) stem cells.Stem Cell Reports. 2015 May 12;4(5):759-67. doi: 10.1016/j.stemcr.2015.03.003. Epub 2015 Apr 16. Stem Cell Reports. 2015. PMID: 25892522 Free PMC article.
-
Phenotypic landscape of intestinal organoid regeneration.Nature. 2020 Oct;586(7828):275-280. doi: 10.1038/s41586-020-2776-9. Epub 2020 Oct 7. Nature. 2020. PMID: 33029001 Free PMC article.
-
Single-Cell Transcript Profiles Reveal Multilineage Priming in Early Progenitors Derived from Lgr5(+) Intestinal Stem Cells.Cell Rep. 2016 Aug 23;16(8):2053-2060. doi: 10.1016/j.celrep.2016.07.056. Epub 2016 Aug 11. Cell Rep. 2016. PMID: 27524622 Free PMC article.
-
The Anti-Oxidative, Anti-Inflammatory, Anti-Apoptotic, and Anti-Necroptotic Role of Zinc in COVID-19 and Sepsis.Antioxidants (Basel). 2023 Oct 31;12(11):1942. doi: 10.3390/antiox12111942. Antioxidants (Basel). 2023. PMID: 38001795 Free PMC article. Review.
References
-
- Barker N, van Es JH, Kuipers J, Kujala J,P, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Marshman E, Booth C, Potten CS. The intestinal epithelial stem cell. Bioessays. 2002;24:91–98. - PubMed
-
- Cheng H, Leblond CP. Origin, differentiation and renewal of the four main epithelial cell types in the mouse small intestine. I. Columnar cell. Am J Anat. 1974;141:461–479. - PubMed
-
- Bjerknes M, Cheng H. Clonal analysis of mouse intestinal epithelial progenitors. Gastroenterology. 1999;116:7–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

