Homeostatic control of synaptic activity by endogenous adenosine is mediated by adenosine kinase
- PMID: 22997174
- PMCID: PMC3862265
- DOI: 10.1093/cercor/bhs284
Homeostatic control of synaptic activity by endogenous adenosine is mediated by adenosine kinase
Abstract
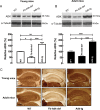
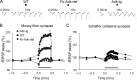
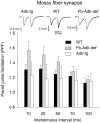
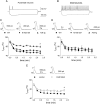
Extracellular adenosine, a key regulator of neuronal excitability, is metabolized by astrocyte-based enzyme adenosine kinase (ADK). We hypothesized that ADK might be an upstream regulator of adenosine-based homeostatic brain functions by simultaneously affecting several downstream pathways. We therefore studied the relationship between ADK expression, levels of extracellular adenosine, synaptic transmission, intrinsic excitability, and brain-derived neurotrophic factor (BDNF)-dependent synaptic actions in transgenic mice underexpressing or overexpressing ADK. We demonstrate that ADK: 1) Critically influences the basal tone of adenosine, evaluated by microelectrode adenosine biosensors, and its release following stimulation; 2) determines the degree of tonic adenosine-dependent synaptic inhibition, which correlates with differential plasticity at hippocampal synapses with low release probability; 3) modulates the age-dependent effects of BDNF on hippocampal synaptic transmission, an action dependent upon co-activation of adenosine A2A receptors; and 4) influences GABAA receptor-mediated currents in CA3 pyramidal neurons. We conclude that ADK provides important upstream regulation of adenosine-based homeostatic function of the brain and that this mechanism is necessary and permissive to synaptic actions of adenosine acting on multiple pathways. These mechanistic studies support previous therapeutic studies and implicate ADK as a promising therapeutic target for upstream control of multiple neuronal signaling pathways crucial for a variety of neurological disorders.
Keywords: GABA; adenosine; brain-derived neurotrophic factor; homeostasis; transgenic mice.
Figures



Similar articles
-
Adenosine Kinase Deficiency in the Brain Results in Maladaptive Synaptic Plasticity.J Neurosci. 2016 Nov 30;36(48):12117-12128. doi: 10.1523/JNEUROSCI.2146-16.2016. J Neurosci. 2016. PMID: 27903722 Free PMC article.
-
The role of extracellular adenosine in regulating mossy fiber synaptic plasticity.J Neurosci. 2005 Mar 16;25(11):2832-7. doi: 10.1523/JNEUROSCI.4260-04.2005. J Neurosci. 2005. PMID: 15772343 Free PMC article.
-
Activation of adenosine A2A receptor facilitates brain-derived neurotrophic factor modulation of synaptic transmission in hippocampal slices.J Neurosci. 2004 Mar 24;24(12):2905-13. doi: 10.1523/JNEUROSCI.4454-03.2004. J Neurosci. 2004. PMID: 15044529 Free PMC article.
-
Adenosine kinase: exploitation for therapeutic gain.Pharmacol Rev. 2013 Apr 16;65(3):906-43. doi: 10.1124/pr.112.006361. Print 2013 Jul. Pharmacol Rev. 2013. PMID: 23592612 Free PMC article. Review.
-
Adenosine kinase: A key regulator of purinergic physiology.Biochem Pharmacol. 2021 May;187:114321. doi: 10.1016/j.bcp.2020.114321. Epub 2020 Nov 6. Biochem Pharmacol. 2021. PMID: 33161022 Free PMC article. Review.
Cited by
-
Basal adenosine modulates the functional properties of AMPA receptors in mouse hippocampal neurons through the activation of A1R A2AR and A3R.Front Cell Neurosci. 2015 Oct 12;9:409. doi: 10.3389/fncel.2015.00409. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26528137 Free PMC article.
-
Adenosine A2A Receptors Shut Down Adenosine A1 Receptor-Mediated Presynaptic Inhibition to Promote Implementation of Hippocampal Long-Term Potentiation.Biomolecules. 2023 Apr 21;13(4):715. doi: 10.3390/biom13040715. Biomolecules. 2023. PMID: 37189461 Free PMC article.
-
From unwitnessed fatality to witnessed rescue: Pharmacologic intervention in sudden unexpected death in epilepsy.Epilepsia. 2016 Jan;57 Suppl 1(Suppl 1):35-45. doi: 10.1111/epi.13236. Epilepsia. 2016. PMID: 26749015 Free PMC article. Review.
-
Connexins and pannexins: At the junction of neuro-glial homeostasis & disease.J Neurosci Res. 2018 Jan;96(1):31-44. doi: 10.1002/jnr.24088. Epub 2017 Jun 5. J Neurosci Res. 2018. PMID: 28580666 Free PMC article. Review.
-
Selective modulation of epileptic tissue by an adenosine A3 receptor-activating drug.Br J Pharmacol. 2024 Dec;181(24):5041-5061. doi: 10.1111/bph.17319. Epub 2024 Sep 19. Br J Pharmacol. 2024. PMID: 39300608
References
-
- Anderson WW, Collingridge GL. The LTP Program: A data acquisition program for on-line analysis of long-term potentiation and other synaptic events. J Neurosci Methods. 2001;108:71–83. doi:10.1016/S0165-0270(01)00374-0. - DOI - PubMed
-
- Aronica E, Zurolo E, Iyer A, de Groot M, Anink J, Carbonell C, van Vliet EA, Baayen JC, Boison D, Gorter JA. Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy. Epilepsia. 2011;52:1645–1655. doi:10.1111/j.1528-1167.2011.03115.x. - DOI - PMC - PubMed
-
- Assaife-Lopes N, Sousa VC, Pereira DB, Ribeiro JA, Chao MV, Sebastião AM. Activation of adenosine A2A receptors induces TrkB translocation and increases BDNF-mediated phospho-TrkB localization in lipid rafts: Implications for neuromodulation. J Neurosci. 2010;30:8468–8480. doi:10.1523/JNEUROSCI.5695-09.2010. - DOI - PMC - PubMed
-
- Boison D. Adenosine augmentation therapies (AATs) for epilepsy: Prospect of cell and gene therapies. Epilepsy Res. 2009;85:131–141. doi:10.1016/j.eplepsyres.2009.03.019. - DOI - PMC - PubMed
-
- Boison D, Chen JF, Fredholm BB. Adenosine signaling and function in glial cells. Cell Death Differ. 2010;17:1071–1082. doi:10.1038/cdd.2009.131. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

