One-step purification of assembly-competent tubulin from diverse eukaryotic sources
- PMID: 22993214
- PMCID: PMC3496613
- DOI: 10.1091/mbc.E12-06-0444
One-step purification of assembly-competent tubulin from diverse eukaryotic sources
Abstract
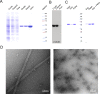
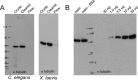
We have developed a protocol that allows rapid and efficient purification of native, active tubulin from a variety of species and tissue sources by affinity chromatography. The affinity matrix comprises a bacterially expressed, recombinant protein, the TOG1/2 domains from Saccharomyces cerevisiae Stu2, covalently coupled to a Sepharose support. The resin has a high capacity to specifically bind tubulin from clarified crude cell extracts, and, after washing, highly purified tubulin can be eluted under mild conditions. The eluted tubulin is fully functional and can be efficiently assembled into microtubules. The method eliminates the need to use heterologous systems for the study of microtubule-associated proteins and motor proteins, which has been a major issue in microtubule-related research.
Figures




Similar articles
-
Purification of native M. vogae and H. contortus tubulin by TOG affinity chromatography.Exp Parasitol. 2017 Nov;182:37-44. doi: 10.1016/j.exppara.2017.09.025. Epub 2017 Sep 20. Exp Parasitol. 2017. PMID: 28942049
-
A tethered delivery mechanism explains the catalytic action of a microtubule polymerase.Elife. 2014 Aug 5;3:e03069. doi: 10.7554/eLife.03069. Elife. 2014. PMID: 25097237 Free PMC article.
-
Affinity Purification and Characterization of Functional Tubulin from Cell Suspension Cultures of Arabidopsis and Tobacco.Plant Physiol. 2016 Mar;170(3):1189-205. doi: 10.1104/pp.15.01173. Epub 2016 Jan 8. Plant Physiol. 2016. PMID: 26747285 Free PMC article.
-
Affinity purification of tubulin from plant materials.Methods Cell Biol. 2020;160:263-280. doi: 10.1016/bs.mcb.2020.06.002. Epub 2020 Jul 22. Methods Cell Biol. 2020. PMID: 32896321
-
Gamma-tubulin at ten: progress and prospects.Cell Struct Funct. 1999 Oct;24(5):365-72. doi: 10.1247/csf.24.365. Cell Struct Funct. 1999. PMID: 15216894 Review.
Cited by
-
Mutations in Human Tubulin Proximal to the Kinesin-Binding Site Alter Dynamic Instability at Microtubule Plus- and Minus-Ends.Dev Cell. 2016 Apr 4;37(1):72-84. doi: 10.1016/j.devcel.2016.03.003. Dev Cell. 2016. PMID: 27046833 Free PMC article.
-
Displacement-weighted velocity analysis of gliding assays reveals that Chlamydomonas axonemal dynein preferentially moves conspecific microtubules.Biophys J. 2013 May 7;104(9):1989-98. doi: 10.1016/j.bpj.2013.03.041. Biophys J. 2013. PMID: 23663842 Free PMC article.
-
In Vitro Microtubule Dynamics Assays Using Dark-Field Microscopy.Methods Mol Biol. 2020;2101:39-51. doi: 10.1007/978-1-0716-0219-5_4. Methods Mol Biol. 2020. PMID: 31879897 Free PMC article.
-
Intrinsically disordered tubulin tails: complex tuners of microtubule functions?Semin Cell Dev Biol. 2015 Jan;37:11-9. doi: 10.1016/j.semcdb.2014.09.026. Epub 2014 Oct 13. Semin Cell Dev Biol. 2015. PMID: 25307498 Free PMC article. Review.
-
Crystal structure of tubulin tyrosine ligase-like 3 reveals essential architectural elements unique to tubulin monoglycylases.Proc Natl Acad Sci U S A. 2017 Jun 20;114(25):6545-6550. doi: 10.1073/pnas.1617286114. Epub 2017 Jun 2. Proc Natl Acad Sci U S A. 2017. PMID: 28576883 Free PMC article.
References
-
- Akhmanova A, Steinmetz MO. Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat Rev. Mol Cell Biol. 2008;9:309–322. - PubMed
-
- Al-Bassam J, Larsen N, Hyman A, Harrison S. Crystal structure of a TOG domain: conserved features of XMAP215/Dis1-family TOG domains and implications for tubulin binding. Structure. 2007;15:355–362. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

