A herpes simplex virus 2 glycoprotein D mutant generated by bacterial artificial chromosome mutagenesis is severely impaired for infecting neuronal cells and infects only Vero cells expressing exogenous HVEM
- PMID: 22993162
- PMCID: PMC3497698
- DOI: 10.1128/JVI.01055-12
A herpes simplex virus 2 glycoprotein D mutant generated by bacterial artificial chromosome mutagenesis is severely impaired for infecting neuronal cells and infects only Vero cells expressing exogenous HVEM
Abstract
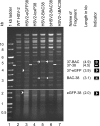
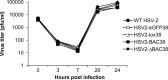
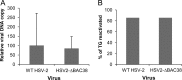
We constructed a herpes simplex virus 2 (HSV-2) bacterial artificial chromosome (BAC) clone, bHSV2-BAC38, which contains full-length HSV-2 inserted into a BAC vector. Unlike previously reported HSV-2 BAC clones, the virus genome inserted into this BAC clone has no known gene disruptions. Virus derived from the BAC clone had a wild-type phenotype for growth in vitro and for acute infection, latency, and reactivation in mice. HVEM, expressed on epithelial cells and lymphocytes, and nectin-1, expressed on neurons and epithelial cells, are the two principal receptors used by HSV to enter cells. We used the HSV-2 BAC clone to construct an HSV-2 glycoprotein D mutant (HSV2-gD27) with point mutations in amino acids 215, 222, and 223, which are critical for the interaction of gD with nectin-1. HSV2-gD27 infected cells expressing HVEM, including a human epithelial cell line. However, the virus lost the ability to infect cells expressing only nectin-1, including neuronal cell lines, and did not infect ganglia in mice. Surprisingly, we found that HSV2-gD27 could not infect Vero cells unless we transduced the cells with a retrovirus expressing HVEM. High-level expression of HVEM in Vero cells also resulted in increased syncytia and enhanced cell-to-cell spread in cells infected with wild-type HSV-2. The inability of the HSV2-gD27 mutant to infect neuronal cells in vitro or sensory ganglia in mice after intramuscular inoculation suggests that this HSV-2 mutant might be an attractive candidate for a live attenuated HSV-2 vaccine.
Figures

Similar articles
-
B Virus (Macacine Herpesvirus 1) Divergence: Variations in Glycoprotein D from Clinical and Laboratory Isolates Diversify Virus Entry Strategies.J Virol. 2016 Sep 29;90(20):9420-32. doi: 10.1128/JVI.00799-16. Print 2016 Oct 15. J Virol. 2016. PMID: 27512063 Free PMC article.
-
A Herpes Simplex Virus 2 (HSV-2) gD Mutant Impaired for Neural Tropism Is Superior to an HSV-2 gD Subunit Vaccine To Protect Animals from Challenge with HSV-2.J Virol. 2015 Nov 11;90(1):562-74. doi: 10.1128/JVI.01845-15. Print 2016 Jan 1. J Virol. 2015. PMID: 26559846 Free PMC article.
-
An HSV-1 gD mutant virus as an entry-impaired live virus vaccine.Vaccine. 2008 Feb 26;26(9):1195-203. doi: 10.1016/j.vaccine.2007.12.032. Epub 2008 Jan 14. Vaccine. 2008. PMID: 18243431 Free PMC article.
-
Herpesvirus Entry Mediator and Ocular Herpesvirus Infection: More than Meets the Eye.J Virol. 2017 Jun 9;91(13):e00115-17. doi: 10.1128/JVI.00115-17. Print 2017 Jul 1. J Virol. 2017. PMID: 28404853 Free PMC article. Review.
-
Herpes simplex virus infects most cell types in vitro: clues to its success.Virol J. 2011 Oct 26;8:481. doi: 10.1186/1743-422X-8-481. Virol J. 2011. PMID: 22029482 Free PMC article. Review.
Cited by
-
Pharmacological Induction of Heme Oxygenase-1 Impairs Nuclear Accumulation of Herpes Simplex Virus Capsids upon Infection.Front Microbiol. 2017 Oct 31;8:2108. doi: 10.3389/fmicb.2017.02108. eCollection 2017. Front Microbiol. 2017. PMID: 29163402 Free PMC article.
-
Combinatorial Herpes Simplex Vaccine Strategies: From Bedside to Bench and Back.Front Immunol. 2022 Apr 25;13:849515. doi: 10.3389/fimmu.2022.849515. eCollection 2022. Front Immunol. 2022. PMID: 35547736 Free PMC article. Review.
-
Displacement of the C terminus of herpes simplex virus gD is sufficient to expose the fusion-activating interfaces on gD.J Virol. 2013 Dec;87(23):12656-66. doi: 10.1128/JVI.01727-13. Epub 2013 Sep 18. J Virol. 2013. PMID: 24049165 Free PMC article.
-
US6 Gene Deletion in Herpes Simplex Virus Type 2 Enhances Dendritic Cell Function and T Cell Activation.Front Immunol. 2017 Nov 10;8:1523. doi: 10.3389/fimmu.2017.01523. eCollection 2017. Front Immunol. 2017. PMID: 29176979 Free PMC article.
-
Herpes Simplex Vaccines: Prospects of Live-attenuated HSV Vaccines to Combat Genital and Ocular infections.Curr Clin Microbiol Rep. 2015 Sep 1;2(3):125-136. doi: 10.1007/s40588-015-0020-4. Epub 2015 Jul 1. Curr Clin Microbiol Rep. 2015. PMID: 27114893 Free PMC article.
References
-
- Brugha R, Keersmaekers K, Renton A, Meheus A. 1997. Genital herpes infection: a review. Int. J. Epidemiol. 26:698–709 - PubMed
-
- Carfi A, et al. 2002. Crystallization and preliminary diffraction studies of the ectodomain of the envelope glycoprotein D from herpes simplex virus 1 alone and in complex with the ectodomain of the human receptor HveA. Acta Crystallogr. D Biol. Crystallogr. 58:836–838 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

