Engineering neonatal Fc receptor-mediated recycling and transcytosis in recombinant proteins by short terminal peptide extensions
- PMID: 22991460
- PMCID: PMC3479544
- DOI: 10.1073/pnas.1208857109
Engineering neonatal Fc receptor-mediated recycling and transcytosis in recombinant proteins by short terminal peptide extensions
Abstract
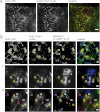
The importance of therapeutic recombinant proteins in medicine has led to a variety of tactics to increase their circulation time or to enable routes of administration other than injection. One clinically successful tactic to improve both protein circulation and delivery is to fuse the Fc domain of IgG to therapeutic proteins so that the resulting fusion proteins interact with the human neonatal Fc receptor (FcRn). As an alternative to grafting the high molecular weight Fc domain to therapeutic proteins, we have modified their N and/or C termini with a short peptide sequence that interacts with FcRn. Our strategy was motivated by results [Mezo AR, et al. (2008) Proc Natl Acad Sci USA 105:2337-2342] that identified peptides that compete with human IgG for FcRn. The small size and simple structure of the FcRn-binding peptide (FcBP) allows for expression of FcBP fusion proteins in Escherichia coli and results in their pH-dependent binding to FcRn with an affinity comparable to that of IgG. The FcBP fusion proteins are internalized, recycled, and transcytosed across cell monolayers that express FcRn. This strategy has the potential to improve protein transport across epithelial barriers, which could lead to noninvasive administration and also enable longer half-lives of therapeutic proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
Enhanced FcRn-dependent transepithelial delivery of IgG by Fc-engineering and polymerization.J Control Release. 2016 Feb 10;223:42-52. doi: 10.1016/j.jconrel.2015.12.033. Epub 2015 Dec 21. J Control Release. 2016. PMID: 26718855
-
Development of a label-free FcRn-mediated transcytosis assay for in vitro characterization of FcRn interactions with therapeutic antibodies and Fc-fusion proteins.J Immunol Methods. 2018 Nov;462:101-105. doi: 10.1016/j.jim.2018.07.004. Epub 2018 Jul 18. J Immunol Methods. 2018. PMID: 30030147
-
Combined glyco- and protein-Fc engineering simultaneously enhance cytotoxicity and half-life of a therapeutic antibody.MAbs. 2014 Mar-Apr;6(2):422-36. doi: 10.4161/mabs.27854. Epub 2014 Jan 15. MAbs. 2014. PMID: 24492301 Free PMC article.
-
Neonatal Fc receptor (FcRn): a novel target for therapeutic antibodies and antibody engineering.J Drug Target. 2014 May;22(4):269-78. doi: 10.3109/1061186X.2013.875030. Epub 2014 Jan 9. J Drug Target. 2014. PMID: 24404896 Review.
-
Current strategies in antibody engineering: Fc engineering and pH-dependent antigen binding, bispecific antibodies and antibody drug conjugates.Biotechnol J. 2012 Dec;7(12):1444-50. doi: 10.1002/biot.201200250. Epub 2012 Nov 1. Biotechnol J. 2012. PMID: 23125076 Review.
Cited by
-
Rotavirus as an Expression Platform of Domains of the SARS-CoV-2 Spike Protein.Vaccines (Basel). 2021 May 3;9(5):449. doi: 10.3390/vaccines9050449. Vaccines (Basel). 2021. PMID: 34063562 Free PMC article.
-
Fc-fusion proteins and FcRn: structural insights for longer-lasting and more effective therapeutics.Crit Rev Biotechnol. 2015 Jun;35(2):235-54. doi: 10.3109/07388551.2013.834293. Epub 2013 Oct 24. Crit Rev Biotechnol. 2015. PMID: 24156398 Free PMC article. Review.
-
In Translation: FcRn across the Therapeutic Spectrum.Int J Mol Sci. 2021 Mar 17;22(6):3048. doi: 10.3390/ijms22063048. Int J Mol Sci. 2021. PMID: 33802650 Free PMC article. Review.
-
Role of critical elements in botulinum neurotoxin complex in toxin routing across intestinal and bronchial barriers.PLoS One. 2018 Jul 5;13(7):e0199524. doi: 10.1371/journal.pone.0199524. eCollection 2018. PLoS One. 2018. PMID: 29975725 Free PMC article.
-
Recombinant human endostatin as a potential anti-angiogenic agent: therapeutic perspective and current status.Med Oncol. 2023 Dec 21;41(1):24. doi: 10.1007/s12032-023-02245-w. Med Oncol. 2023. PMID: 38123873 Review.
References
-
- Kontermann RE. Strategies for extended serum half-life of protein therapeutics. Curr Opin Biotechnol. 2011;22:868–876. - PubMed
-
- Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–558. - PubMed
-
- Roopenian DC, Akilesh S. FcRn: The neonatal Fc receptor comes of age. Nat Rev Immunol. 2007;7:715–725. - PubMed
-
- Abuchowski A, McCoy JR, Palczuk NC, van Es T, Davis FF. Effect of covalent attachment of polyethylene glycol on immunogenicity and circulating life of bovine liver catalase. J Biol Chem. 1977;252:3582–3586. - PubMed
-
- Alconcel SNS, Baas AS, Maynard HD. FDA-approved poly(ethylene glycol)–protein conjugate drugs. Polymer Chem. 2011;2:1442–1448.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

