Is ZD7288 a selective blocker of hyperpolarization-activated cyclic nucleotide-gated channel currents?
- PMID: 22989944
- PMCID: PMC3536728
- DOI: 10.4161/chan.22209
Is ZD7288 a selective blocker of hyperpolarization-activated cyclic nucleotide-gated channel currents?
Abstract
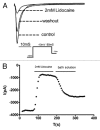
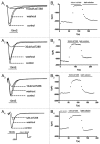
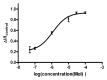
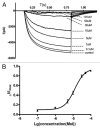
ZD7288 has been widely used as a tool in the study of hyperpolarization-activated cyclic nucleotide-gated channels (HCN channels), and to test the relationships between HCN channels and heart and brain function. ZD7288 is widely considered a selective blocker of HCN currents. Here we show that ZD7288 inhibits not only HCN channel currents, but also Na(+) currents in DRG neurons and ZD7288 was confirmed to inhibit Na(+) current in HEK293 cells transfected with Na(v)1.4 plasmids. Thus our findings challenge the view that ZD7288 is a selective blocker of HCN channels. Conclusions about the role of NCN channels in neuronal function should be re-evaluated if based exclusively on the effect of ZD7288.
Figures

Similar articles
-
Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain.Sheng Li Xue Bao. 2008 Oct 25;60(5):579-80. Sheng Li Xue Bao. 2008. PMID: 18958363
-
Hyperpolarization-activated cyclic nucleotide-gated cation channel subtypes differentially modulate the excitability of murine small intestinal afferents.World J Gastroenterol. 2012 Feb 14;18(6):522-31. doi: 10.3748/wjg.v18.i6.522. World J Gastroenterol. 2012. PMID: 22363118 Free PMC article.
-
Septal infusions of the hyperpolarization-activated cyclic nucleotide-gated channel (HCN-channel) blocker ZD7288 impair spontaneous alternation but not inhibitory avoidance.Behav Neurosci. 2008 Jun;122(3):549-56. doi: 10.1037/0735-7044.122.3.549. Behav Neurosci. 2008. PMID: 18513125
-
Hyperpolarization-activated and cyclic nucleotide-gated channel proteins as emerging new targets in neuropathic pain.Rev Neurosci. 2019 Jul 26;30(6):639-649. doi: 10.1515/revneuro-2018-0094. Rev Neurosci. 2019. PMID: 30768426 Review.
-
Hyperpolarization-activated cyclic nucleotide-gated (HCN) channels and pain.Curr Pharm Des. 2009;15(15):1767-72. doi: 10.2174/138161209788186281. Curr Pharm Des. 2009. PMID: 19442189 Review.
Cited by
-
Sedative Properties of Dexmedetomidine Are Mediated Independently from Native Thalamic Hyperpolarization-Activated Cyclic Nucleotide-Gated Channel Function at Clinically Relevant Concentrations.Int J Mol Sci. 2022 Dec 28;24(1):519. doi: 10.3390/ijms24010519. Int J Mol Sci. 2022. PMID: 36613961 Free PMC article.
-
Characterization of Dmrt3-Derived Neurons Suggest a Role within Locomotor Circuits.J Neurosci. 2019 Mar 6;39(10):1771-1782. doi: 10.1523/JNEUROSCI.0326-18.2018. Epub 2018 Dec 21. J Neurosci. 2019. PMID: 30578339 Free PMC article.
-
Volatile anesthetics inhibit presynaptic cGMP signaling to depress presynaptic excitability in rat hippocampal neurons.Neuropharmacology. 2023 Dec 1;240:109705. doi: 10.1016/j.neuropharm.2023.109705. Epub 2023 Sep 6. Neuropharmacology. 2023. PMID: 37683886 Free PMC article.
-
Accumulation of K+ in the synaptic cleft modulates activity by influencing both vestibular hair cell and calyx afferent in the turtle.J Physiol. 2017 Feb 1;595(3):777-803. doi: 10.1113/JP273060. Epub 2016 Nov 4. J Physiol. 2017. PMID: 27633787 Free PMC article.
-
Dopamine Deficiency Reduces Striatal Cholinergic Interneuron Function in Models of Parkinson's Disease.Neuron. 2019 Sep 25;103(6):1056-1072.e6. doi: 10.1016/j.neuron.2019.06.013. Epub 2019 Jul 16. Neuron. 2019. PMID: 31324539 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
