INF2 promotes the formation of detyrosinated microtubules necessary for centrosome reorientation in T cells
- PMID: 22986496
- PMCID: PMC3444772
- DOI: 10.1083/jcb.201202137
INF2 promotes the formation of detyrosinated microtubules necessary for centrosome reorientation in T cells
Abstract
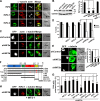
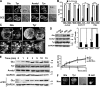
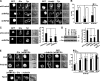
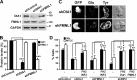
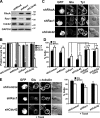
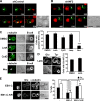
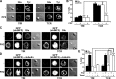
T cell antigen receptor-proximal signaling components, Rho-family GTPases, and formin proteins DIA1 and FMNL1 have been implicated in centrosome reorientation to the immunological synapse of T lymphocytes. However, the role of these molecules in the reorientation process is not yet defined. Here we find that a subset of microtubules became rapidly stabilized and that their α-tubulin subunit posttranslationally detyrosinated after engagement of the T cell receptor. Formation of stabilized, detyrosinated microtubules required the formin INF2, which was also found to be essential for centrosome reorientation, but it occurred independently of T cell receptor-induced massive tyrosine phosphorylation. The FH2 domain, which was mapped as the INF2 region involved in centrosome repositioning, was able to mediate the formation of stable, detyrosinated microtubules and to restore centrosome translocation in DIA1-, FMNL1-, Rac1-, and Cdc42-deficient cells. Further experiments indicated that microtubule stabilization was required for centrosome polarization. Our work identifies INF2 and stable, detyrosinated microtubules as central players in centrosome reorientation in T cells.
Figures
Similar articles
-
Centrosome polarization in T cells: a task for formins.Front Immunol. 2013 Jul 11;4:191. doi: 10.3389/fimmu.2013.00191. eCollection 2013. Front Immunol. 2013. PMID: 23874337 Free PMC article.
-
Formins regulate the actin-related protein 2/3 complex-independent polarization of the centrosome to the immunological synapse.Immunity. 2007 Feb;26(2):177-90. doi: 10.1016/j.immuni.2007.01.008. Immunity. 2007. PMID: 17306570 Free PMC article.
-
Formin INF2 regulates MAL-mediated transport of Lck to the plasma membrane of human T lymphocytes.Blood. 2010 Dec 23;116(26):5919-29. doi: 10.1182/blood-2010-08-300665. Epub 2010 Sep 29. Blood. 2010. PMID: 20881207
-
Formin proteins in megakaryocytes and platelets: regulation of actin and microtubule dynamics.Platelets. 2019;30(1):23-30. doi: 10.1080/09537104.2018.1481937. Epub 2018 Jun 18. Platelets. 2019. PMID: 29913076 Free PMC article. Review.
-
Reforming the Barrier: The Role of Formins in Wound Repair.Cells. 2022 Sep 6;11(18):2779. doi: 10.3390/cells11182779. Cells. 2022. PMID: 36139355 Free PMC article. Review.
Cited by
-
From lipid second messengers to molecular motors: microtubule-organizing center reorientation in T cells.Immunol Rev. 2013 Nov;256(1):95-106. doi: 10.1111/imr.12116. Immunol Rev. 2013. PMID: 24117815 Free PMC article. Review.
-
Collective dynamics of actin and microtubule and its crosstalk mediated by FHDC1.Front Cell Dev Biol. 2024 Mar 19;11:1261117. doi: 10.3389/fcell.2023.1261117. eCollection 2023. Front Cell Dev Biol. 2024. PMID: 38567385 Free PMC article.
-
Differential Toxicity of mDia Formin-Directed Functional Agonists and Antagonists in Developing Zebrafish.Front Pharmacol. 2018 Apr 10;9:340. doi: 10.3389/fphar.2018.00340. eCollection 2018. Front Pharmacol. 2018. PMID: 29692731 Free PMC article.
-
Mycophenolic acid inhibits migration and invasion of gastric cancer cells via multiple molecular pathways.PLoS One. 2013 Nov 15;8(11):e81702. doi: 10.1371/journal.pone.0081702. eCollection 2013. PLoS One. 2013. PMID: 24260584 Free PMC article.
-
Molecular mechanisms and functional implications of polarized actin remodeling at the T cell immunological synapse.Cell Mol Life Sci. 2015 Feb;72(3):537-556. doi: 10.1007/s00018-014-1760-7. Epub 2014 Oct 30. Cell Mol Life Sci. 2015. PMID: 25355055 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

