EphA/ephrin-A signaling is critically involved in region-specific apoptosis during early brain development
- PMID: 22976838
- PMCID: PMC3524646
- DOI: 10.1038/cdd.2012.121
EphA/ephrin-A signaling is critically involved in region-specific apoptosis during early brain development
Abstract
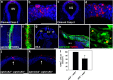
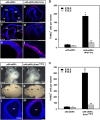
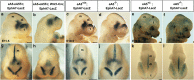
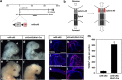
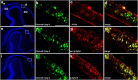
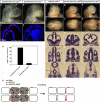
EphAs and ephrin-As have been implicated in the morphogenesis of the developing brain. We found that EphA7 and ephrin-A5 are coexpressed in the dorsal midline (DM) of the diencephalon and anterior mesencephalon. Interestingly, programmed cell death (PCD) of the neural epithelial cells normally found in this region was reduced in ephrin-A5/ephrin-A2 dual-deficient embryos. In contrast, in vivo expression of ephrin-A5-Fc or full-length ephrin-A5 strongly induced apoptosis in neural epithelial cells and was accompanied by severe brain malformation during embryonic development. Expression of ephrinA5-Fc correlated with apoptosis of EphA7-expressing cells, whereas null mutation of ephrin-A5 resulted in the converse phenotype. Importantly, null mutation of caspase-3 or endogenous ephrin-A5 attenuated the PCD induced by ectopically overexpressed ephrin-A5. Together, our results suggest that brain region-specific PCD may occur in a region where EphAs cluster with neighboring ephrin-As through cell-cell contact.
Figures


Similar articles
-
Expression of EphA8-Fc in transgenic mouse embryos induces apoptosis of neural epithelial cells during brain development.Dev Neurobiol. 2013 Sep;73(9):702-12. doi: 10.1002/dneu.22092. Epub 2013 Jun 29. Dev Neurobiol. 2013. PMID: 23696555
-
Effects of altered ephrin-A5 and EphA4/EphA7 expression on tumor growth in a medulloblastoma mouse model.J Hematol Oncol. 2015 Sep 7;8:105. doi: 10.1186/s13045-015-0202-9. J Hematol Oncol. 2015. PMID: 26345456 Free PMC article.
-
EphrinA5-EphA7 complex induces apoptotic cell death via TNFR1.Mol Cells. 2013 May;35(5):450-5. doi: 10.1007/s10059-013-0072-3. Epub 2013 May 3. Mol Cells. 2013. PMID: 23657875 Free PMC article.
-
In vivo expression of ephrinA5-Fc in mice results in cephalic neural crest agenesis and craniofacial abnormalities.Mol Cells. 2014 Jan;37(1):59-65. doi: 10.14348/molcells.2014.2279. Epub 2014 Jan 27. Mol Cells. 2014. PMID: 24552711 Free PMC article.
-
The role of EphA7 in different tumors.Clin Transl Oncol. 2022 Jul;24(7):1274-1289. doi: 10.1007/s12094-022-02783-1. Epub 2022 Feb 2. Clin Transl Oncol. 2022. PMID: 35112312 Review.
Cited by
-
Ephrin-B2 paces neuronal production in the developing neocortex.BMC Dev Biol. 2020 May 13;20(1):12. doi: 10.1186/s12861-020-00215-3. BMC Dev Biol. 2020. PMID: 32404061 Free PMC article.
-
The phenotypic spectrum of proximal 6q deletions based on a large cohort derived from social media and literature reports.Eur J Hum Genet. 2018 Oct;26(10):1478-1489. doi: 10.1038/s41431-018-0172-9. Epub 2018 Jun 8. Eur J Hum Genet. 2018. PMID: 29904178 Free PMC article.
-
Sprint Interval Training Improves Brain-Derived Neurotropic Factor-Induced Benefits in Brain Health-A Possible Molecular Signaling Intervention.Biology (Basel). 2024 Jul 26;13(8):562. doi: 10.3390/biology13080562. Biology (Basel). 2024. PMID: 39194500 Free PMC article. Review.
-
Multiomics integration-based immunological characterizations of adamantinomatous craniopharyngioma in relation to keratinization.Cell Death Dis. 2024 Jun 21;15(6):439. doi: 10.1038/s41419-024-06840-1. Cell Death Dis. 2024. PMID: 38906852 Free PMC article.
-
Ephrin reverse signaling mediates palatal fusion and epithelial-to-mesenchymal transition independently of Tgfß3.J Cell Physiol. 2015 Dec;230(12):2961-72. doi: 10.1002/jcp.25025. J Cell Physiol. 2015. PMID: 25893671 Free PMC article.
References
-
- Homma S, Yaginuma H, Oppenheim RW. Programmed cell death during the earliest stages of spinal cord development in the chick embryo: a possible means of early phenotypic selection. J Comp Neurol. 1994;345:377–395. - PubMed
-
- de la Rosa EJ, de Pablo F. Cell death in early neural development: beyond the neurotrophic theory. Trends Neurosci. 2000;23:454–458. - PubMed
-
- Kuan CY, Roth KA, Flavell RA, Rakic P. Mechanisms of programmed cell death in the developing brain. Trends Neurosci. 2000;23:291–297. - PubMed
-
- Yeo W, Gautier J. Early neural cell death: dying to become neurons. Dev Biol. 2004;274:233–244. - PubMed
-
- Buss RR, Sun W, Oppenheim RW. Adaptive roles of programmed cell death during nervous system development. Annu Rev Neurosci. 2006;29:1–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

