Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells
- PMID: 22966020
- PMCID: PMC4337849
- DOI: 10.1158/1078-0432.CCR-12-0319
Suppression of human glioma xenografts with second-generation IL13R-specific chimeric antigen receptor-modified T cells
Abstract
Purpose: Glioblastoma multiforme (GBM) remains highly incurable, with frequent recurrences after standard therapies of maximal surgical resection, radiation, and chemotherapy. To address the need for new treatments, we have undertaken a chimeric antigen receptor (CAR) "designer T cell" (dTc) immunotherapeutic strategy by exploiting interleukin (IL)13 receptor α-2 (IL13Rα2) as a GBM-selective target.
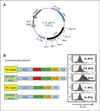
Experimental design: We tested a second-generation IL13 "zetakine" CAR composed of a mutated IL13 extracellular domain linked to intracellular signaling elements of the CD28 costimulatory molecule and CD3ζ. The aim of the mutation (IL13.E13K.R109K) was to enhance selectivity of the CAR for recognition and killing of IL13Rα2(+) GBMs while sparing normal cells bearing the composite IL13Rα1/IL4Rα receptor.
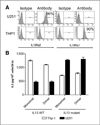
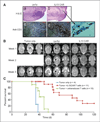
Results: Our aim was partially realized with improved recognition of tumor and reduced but persisting activity against normal tissue IL13Rα1(+) cells by the IL13.E13K.R109K CAR. We show that these IL13 dTcs were efficient in killing IL13Rα2(+) glioma cell targets with abundant secretion of cytokines IL2 and IFNγ, and they displayed enhanced tumor-induced expansion versus control unmodified T cells in vitro. In an in vivo test with a human glioma xenograft model, single intracranial injections of IL13 dTc into tumor sites resulted in marked increases in animal survivals.
Conclusions: These data raise the possibility of immune targeting of diffusely invasive GBM cells either via dTc infusion into resection cavities to prevent GBM recurrence or via direct stereotactic injection of dTcs to suppress inoperable or recurrent tumors. Systemic administration of these IL13 dTc could be complicated by reaction against normal tissues expressing IL13Ra1.
©2012 AACR.
Conflict of interest statement
No potential conflicts of interest were disclosed.
Figures


Comment in
-
Model T muscle CARs can treat brain tumors.Clin Cancer Res. 2012 Nov 1;18(21):5834-6. doi: 10.1158/1078-0432.CCR-12-2627. Epub 2012 Sep 27. Clin Cancer Res. 2012. PMID: 23019306
Similar articles
-
Chimeric Antigen Receptor T Cells With Modified Interleukin-13 Preferentially Recognize IL13Rα2 and Suppress Malignant Glioma: A Preclinical Study.Front Immunol. 2021 Nov 8;12:715000. doi: 10.3389/fimmu.2021.715000. eCollection 2021. Front Immunol. 2021. PMID: 34819930 Free PMC article.
-
T cells redirected to interleukin-13Rα2 with interleukin-13 mutein--chimeric antigen receptors have anti-glioma activity but also recognize interleukin-13Rα1.Cytotherapy. 2014 Aug;16(8):1121-31. doi: 10.1016/j.jcyt.2014.02.012. Epub 2014 May 16. Cytotherapy. 2014. PMID: 24841514 Free PMC article.
-
Inclusion of 4-1BB Costimulation Enhances Selectivity and Functionality of IL13Rα2-Targeted Chimeric Antigen Receptor T Cells.Cancer Res Commun. 2023 Jan 17;3(1):66-79. doi: 10.1158/2767-9764.CRC-22-0185. eCollection 2023 Jan. Cancer Res Commun. 2023. PMID: 36968221 Free PMC article.
-
Current progress in chimeric antigen receptor T cell therapy for glioblastoma multiforme.Cancer Med. 2021 Aug;10(15):5019-5030. doi: 10.1002/cam4.4064. Epub 2021 Jun 19. Cancer Med. 2021. PMID: 34145792 Free PMC article. Review.
-
Chimeric Antigen Receptor T-Cell Therapy in Glioblastoma: Current and Future.Front Immunol. 2020 Nov 3;11:594271. doi: 10.3389/fimmu.2020.594271. eCollection 2020. Front Immunol. 2020. PMID: 33224149 Free PMC article. Review.
Cited by
-
Quo Vadis-Do Immunotherapies Have a Role in Glioblastoma?Curr Treat Options Neurol. 2018 Apr 18;20(5):14. doi: 10.1007/s11940-018-0499-0. Curr Treat Options Neurol. 2018. PMID: 29666934 Review.
-
Chimaeric antigen receptor T-cell therapy for tumour immunotherapy.Biosci Rep. 2017 Jan 27;37(1):BSR20160332. doi: 10.1042/BSR20160332. Print 2017 Feb 28. Biosci Rep. 2017. PMID: 28053197 Free PMC article. Review.
-
Impact of temozolomide on immune response during malignant glioma chemotherapy.Clin Dev Immunol. 2012;2012:831090. doi: 10.1155/2012/831090. Epub 2012 Oct 24. Clin Dev Immunol. 2012. PMID: 23133490 Free PMC article. Review.
-
Silencing of IL13RA2 promotes partial epithelial-mesenchymal transition in hepatocellular carcinoma via ERK signaling pathway activation.FEBS Open Bio. 2020 Feb;10(2):229-236. doi: 10.1002/2211-5463.12774. Epub 2020 Jan 10. FEBS Open Bio. 2020. PMID: 31823484 Free PMC article.
-
A novel TanCAR targeting IL13Rα2 and EphA2 for enhanced glioblastoma therapy.Mol Ther Oncolytics. 2022 Feb 20;24:729-741. doi: 10.1016/j.omto.2022.02.012. eCollection 2022 Mar 17. Mol Ther Oncolytics. 2022. PMID: 35317513 Free PMC article.
References
-
- DeAngelis LM. Brain tumors. N Engl J Med. 2001;344:114–123. - PubMed
-
- Chang SM, Parney IF, Huang W, Anderson FAJ, Asher AL, Bernstein M, et al. Glioma outcomes project investigators patterns of care for adults with newly diagnosed malignant glioma. JAMA. 2005;293:2469–2470. - PubMed
-
- Attenello FJ, Mukherjee D, Datoo G, McGirt MJ, Bohan E, Weingart JD, et al. BCNU wafer in surgical treatment of glioma. Ann Surg Oncol. 2008;15:2887–2893. - PubMed
-
- Facoetti A, Nano R, Zelini P, Morbini P, Benericetti E, Ceroni M, et al. Human leukocyte antigen and antigen processing machinery component defects in astrocytic tumors. Clin Cancer Res. 2005;11:8304–8311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

