The prenyl-binding protein PrBP/δ: a chaperone participating in intracellular trafficking
- PMID: 22960045
- PMCID: PMC3514561
- DOI: 10.1016/j.visres.2012.08.013
The prenyl-binding protein PrBP/δ: a chaperone participating in intracellular trafficking
Abstract
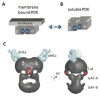
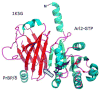
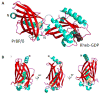
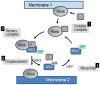
Expressed ubiquitously, PrBP/δ functions as chaperone/co-factor in the transport of a subset of prenylated proteins. PrBP/δ features an immunoglobulin-like β-sandwich fold for lipid binding, and interacts with diverse partners. PrBP/δ binds both C-terminal C15 and C20 prenyl side chains of phototransduction polypeptides and small GTP-binding (G) proteins of the Ras superfamily. PrBP/δ also interacts with the small GTPases, ARL2 and ARL3, which act as release factors (GDFs) for prenylated cargo. Targeted deletion of the mouse Pde6d gene encoding PrBP/δ resulted in impeded trafficking to the outer segments of GRK1 and cone PDE6 which are predicted to be farnesylated and geranylgeranylated, respectively. Rod and cone transducin trafficking was largely unaffected. These trafficking defects produce progressive cone-rod dystrophy in the Pde6d(-/-) mouse.
Copyright © 2012 Elsevier Ltd. All rights reserved.
Figures




Similar articles
-
Deletion of PrBP/delta impedes transport of GRK1 and PDE6 catalytic subunits to photoreceptor outer segments.Proc Natl Acad Sci U S A. 2007 May 22;104(21):8857-62. doi: 10.1073/pnas.0701681104. Epub 2007 May 11. Proc Natl Acad Sci U S A. 2007. PMID: 17496142 Free PMC article.
-
Evaluation of the 17-kDa prenyl-binding protein as a regulatory protein for phototransduction in retinal photoreceptors.J Biol Chem. 2005 Jan 14;280(2):1248-56. doi: 10.1074/jbc.M410475200. Epub 2004 Oct 25. J Biol Chem. 2005. PMID: 15504722 Free PMC article.
-
Membrane protein transport in photoreceptors: the function of PDEδ: the Proctor lecture.Invest Ophthalmol Vis Sci. 2014 Dec 30;55(12):8653-66. doi: 10.1167/iovs.14-16066. Invest Ophthalmol Vis Sci. 2014. PMID: 25550383 Free PMC article.
-
A model for transport of membrane-associated phototransduction polypeptides in rod and cone photoreceptor inner segments.Vision Res. 2008 Feb;48(3):442-52. doi: 10.1016/j.visres.2007.08.020. Epub 2007 Oct 18. Vision Res. 2008. PMID: 17949773 Free PMC article. Review.
-
Sorting of lipidated cargo by the Arl2/Arl3 system.Small GTPases. 2016 Oct;7(4):222-230. doi: 10.1080/21541248.2016.1224454. Epub 2016 Nov 2. Small GTPases. 2016. PMID: 27806215 Free PMC article. Review.
Cited by
-
The interplay between RPGR, PDEδ and Arl2/3 regulate the ciliary targeting of farnesylated cargo.EMBO Rep. 2013 May;14(5):465-72. doi: 10.1038/embor.2013.37. Epub 2013 Apr 5. EMBO Rep. 2013. PMID: 23559067 Free PMC article.
-
PDE6δ-mediated sorting of INPP5E into the cilium is determined by cargo-carrier affinity.Nat Commun. 2016 Apr 11;7:11366. doi: 10.1038/ncomms11366. Nat Commun. 2016. PMID: 27063844 Free PMC article.
-
The ARF GAPs ELMOD1 and ELMOD3 act at the Golgi and cilia to regulate ciliogenesis and ciliary protein traffic.Mol Biol Cell. 2022 Feb 1;33(2):ar13. doi: 10.1091/mbc.E21-09-0443. Epub 2021 Nov 24. Mol Biol Cell. 2022. PMID: 34818063 Free PMC article.
-
Unc119 gene deletion partially rescues the GRK1 transport defect of Pde6d (- /-) cones.Adv Exp Med Biol. 2014;801:487-93. doi: 10.1007/978-1-4614-3209-8_62. Adv Exp Med Biol. 2014. PMID: 24664735 Free PMC article.
-
Cyclase-associated protein 1 (CAP1) is a prenyl-binding partner of Rap1 GTPase.J Biol Chem. 2018 May 18;293(20):7659-7673. doi: 10.1074/jbc.RA118.001779. Epub 2018 Apr 4. J Biol Chem. 2018. PMID: 29618512 Free PMC article.
References
-
- Anant JS, Ong OC, Xie H, Clarke S, O’Brien PJ, Fung BKK. In vivo differential prenylation of retinal cyclic GMP phosphodiesterase catalytic subunits. J Biol Chem. 1992;267:687–690. - PubMed
-
- Aspuria PJ, Tamanoi F. The Rheb family of GTP-binding proteins. Cell Signal. 2004;16:1105–1112. - PubMed
-
- Avruch J, Hara K, Lin Y, Liu M, Long X, Ortiz-Vega S, Yonezawa K. Insulin and amino-acid regulation of mTOR signaling and kinase activity through the Rheb GTPase. Oncogene. 2006;25:6361–6372. - PubMed
-
- Baehr W, Devlin MJ, Applebury ML. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979;254:11669–11677. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

