Efficacy of the β₂-adrenergic receptor is determined by conformational equilibrium in the transmembrane region
- PMID: 22948827
- PMCID: PMC3658005
- DOI: 10.1038/ncomms2046
Efficacy of the β₂-adrenergic receptor is determined by conformational equilibrium in the transmembrane region
Abstract
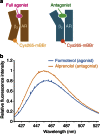
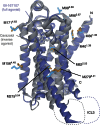
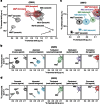
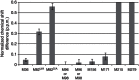
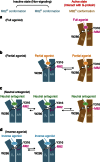
Many drugs that target G-protein-coupled receptors (GPCRs) induce or inhibit their signal transduction with different strengths, which affect their therapeutic properties. However, the mechanism underlying the differences in the signalling levels is still not clear, although several structures of GPCRs complexed with ligands determined by X-ray crystallography are available. Here we utilized NMR to monitor the signals from the methionine residue at position 82 in neutral antagonist- and partial agonist-bound states of β(2)-adrenergic receptor (β(2)AR), which are correlated with the conformational changes of the transmembrane regions upon activation. We show that this residue exists in a conformational equilibrium between the inverse agonist-bound states and the full agonist-bound state, and the population of the latter reflects the signal transduction level in each ligand-bound state. These findings provide insights into the multi-level signalling of β(2)AR and other GPCRs, including the basal activity, and the mechanism of signal transduction mediated by GPCRs.
Figures


Similar articles
-
Structure-Based Prediction of G-Protein-Coupled Receptor Ligand Function: A β-Adrenoceptor Case Study.J Chem Inf Model. 2015 May 26;55(5):1045-61. doi: 10.1021/acs.jcim.5b00066. Epub 2015 May 1. J Chem Inf Model. 2015. PMID: 25848966
-
Allosteric coupling from G protein to the agonist-binding pocket in GPCRs.Nature. 2016 Jul 7;535(7610):182-6. doi: 10.1038/nature18324. Epub 2016 Jun 29. Nature. 2016. PMID: 27362234 Free PMC article.
-
Identifying conformational changes of the beta(2) adrenoceptor that enable accurate prediction of ligand/receptor interactions and screening for GPCR modulators.J Comput Aided Mol Des. 2009 May;23(5):273-88. doi: 10.1007/s10822-008-9257-9. Epub 2009 Jan 16. J Comput Aided Mol Des. 2009. PMID: 19148767 Free PMC article.
-
Structural features of β2 adrenergic receptor: crystal structures and beyond.Mol Cells. 2015;38(2):105-11. doi: 10.14348/molcells.2015.2301. Epub 2014 Dec 24. Mol Cells. 2015. PMID: 25537861 Free PMC article. Review.
-
G protein-coupled receptors--recent advances.Acta Biochim Pol. 2012;59(4):515-29. Epub 2012 Dec 18. Acta Biochim Pol. 2012. PMID: 23251911 Free PMC article. Review.
Cited by
-
Advances in receptor conformation research: the quest for functionally selective conformations focusing on the β2-adrenoceptor.Br J Pharmacol. 2015 Dec;172(23):5477-88. doi: 10.1111/bph.13049. Epub 2015 Feb 27. Br J Pharmacol. 2015. PMID: 25537131 Free PMC article. Review.
-
A Systematic Review of Inverse Agonism at Adrenoceptor Subtypes.Cells. 2020 Aug 19;9(9):1923. doi: 10.3390/cells9091923. Cells. 2020. PMID: 32825009 Free PMC article.
-
Engineering of Challenging G Protein-Coupled Receptors for Structure Determination and Biophysical Studies.Molecules. 2021 Mar 8;26(5):1465. doi: 10.3390/molecules26051465. Molecules. 2021. PMID: 33800379 Free PMC article. Review.
-
Large-scale production and protein engineering of G protein-coupled receptors for structural studies.Front Pharmacol. 2015 Mar 31;6:66. doi: 10.3389/fphar.2015.00066. eCollection 2015. Front Pharmacol. 2015. PMID: 25873898 Free PMC article. Review.
-
Mapping conformational heterogeneity of mitochondrial nucleotide transporter in uninhibited states.Angew Chem Int Ed Engl. 2015 Feb 16;54(8):2436-41. doi: 10.1002/anie.201408417. Epub 2015 Jan 21. Angew Chem Int Ed Engl. 2015. PMID: 25605594 Free PMC article.
References
-
- Lagerström M. C. & Schiöth H. B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat. Rev. Drug Discov. 7, 339–57 (2008). - PubMed
-
- Overington J. P., Al-Lazikani B. & Hopkins A. L. How many drug targets are there? Nat. Rev. Drug Discov. 5, 993–6 (2006). - PubMed
-
- Kobilka B. K. & Deupi X. Conformational complexity of G-protein-coupled receptors. Trends Pharmacol. Sci. 28, 397–406 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

