Relaxin signals through a RXFP1-pERK-nNOS-NO-cGMP-dependent pathway to up-regulate matrix metalloproteinases: the additional involvement of iNOS
- PMID: 22936987
- PMCID: PMC3425563
- DOI: 10.1371/journal.pone.0042714
Relaxin signals through a RXFP1-pERK-nNOS-NO-cGMP-dependent pathway to up-regulate matrix metalloproteinases: the additional involvement of iNOS
Abstract
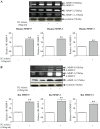
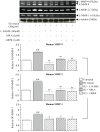
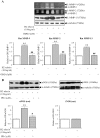
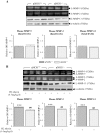
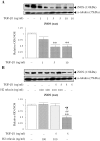
The hormone, relaxin, inhibits aberrant myofibroblast differentiation and collagen deposition by disrupting the TGF-β1/Smad2 axis, via its cognate receptor, Relaxin Family Peptide Receptor 1 (RXFP1), extracellular signal-regulated kinase (ERK)1/2 phosphorylation (pERK) and a neuronal nitric oxide (NO) synthase (nNOS)-NO-cyclic guanosine monophosphate (cGMP)-dependent pathway. However, the signalling pathways involved in its additional ability to increase matrix metalloproteinase (MMP) expression and activity remain unknown. This study investigated the extent to which the NO pathway was involved in human gene-2 (H2) relaxin's ability to positively regulate MMP-1 and its rodent orthologue, MMP-13, MMP-2 and MMP-9 (the main collagen-degrading MMPs) in TGF-β1-stimulated human dermal fibroblasts and primary renal myofibroblasts isolated from injured rats; by gelatin zymography (media) and Western blotting (cell layer). H2 relaxin (10-100 ng/ml) significantly increased MMP-1 (by ~50%), MMP-2 (by ~80%) and MMP-9 (by ~80%) in TGF-β1-stimulated human dermal fibroblasts; and MMP-13 (by ~90%), MMP-2 (by ~130%) and MMP-9 (by ~115%) in rat renal myofibroblasts (all p<0.01 vs untreated cells) over 72 hours. The relaxin-induced up-regulation of these MMPs, however, was significantly blocked by a non-selective NOS inhibitor (L-nitroarginine methyl ester (hydrochloride); L-NAME; 75-100 µM), and specific inhibitors to nNOS (N-propyl-L-arginine; NPLA; 0.2-2 µM), iNOS (1400W; 0.5-1 µM) and guanylyl cyclase (ODQ; 5 µM) (all p<0.05 vs H2 relaxin alone), but not eNOS (L-N-(1-iminoethyl)ornithine dihydrochloride; L-NIO; 0.5-5 µM). However, neither of these inhibitors affected basal MMP expression at the concentrations used. Furthermore, of the NOS isoforms expressed in renal myofibroblasts (nNOS and iNOS), H2 relaxin only stimulated nNOS expression, which in turn, was blocked by the ERK1/2 inhibitor (PD98059; 1 µM). These findings demonstrated that H2 relaxin signals through a RXFP1-pERK-nNOS-NO-cGMP-dependent pathway to mediate its anti-fibrotic actions, and additionally signals through iNOS to up-regulate MMPs; the latter being suppressed by TGF-β1 in myofibroblasts, but released upon H2 relaxin-induced inhibition of the TGF-β1/Smad2 axis.
Conflict of interest statement
Figures

Similar articles
-
Relaxin inhibits renal myofibroblast differentiation via RXFP1, the nitric oxide pathway, and Smad2.FASEB J. 2009 Apr;23(4):1219-29. doi: 10.1096/fj.08-120857. Epub 2008 Dec 10. FASEB J. 2009. PMID: 19073841
-
H3 relaxin demonstrates antifibrotic properties via the RXFP1 receptor.Biochemistry. 2011 Mar 1;50(8):1368-75. doi: 10.1021/bi1013968. Epub 2011 Jan 31. Biochemistry. 2011. PMID: 21229994
-
The anti-fibrotic actions of relaxin are mediated through AT2 R-associated protein phosphatases via RXFP1-AT2 R functional crosstalk in human cardiac myofibroblasts.FASEB J. 2020 Jun;34(6):8217-8233. doi: 10.1096/fj.201902506R. Epub 2020 Apr 16. FASEB J. 2020. PMID: 32297670
-
Understanding relaxin signalling at the cellular level.Mol Cell Endocrinol. 2019 May 1;487:24-33. doi: 10.1016/j.mce.2018.12.017. Epub 2018 Dec 25. Mol Cell Endocrinol. 2019. PMID: 30592984 Review.
-
Site-related Effects of Relaxin in the Gastrointestinal Tract Through Nitric Oxide Signalling: An Updated Report.Curr Protein Pept Sci. 2017;18(12):1254-1262. doi: 10.2174/1389203718666170612104719. Curr Protein Pept Sci. 2017. PMID: 28606038 Review.
Cited by
-
Relaxin decreases the severity of established hepatic fibrosis in mice.Liver Int. 2014 Mar;34(3):416-26. doi: 10.1111/liv.12247. Epub 2013 Jul 21. Liver Int. 2014. PMID: 23870027 Free PMC article.
-
Expression of MMP2, MMP9, TIMP2 and TIMP3 genes in aortic dissection.Exp Ther Med. 2024 Jul 10;28(3):360. doi: 10.3892/etm.2024.12649. eCollection 2024 Sep. Exp Ther Med. 2024. PMID: 39071905 Free PMC article.
-
Relaxin and extracellular matrix remodeling: Mechanisms and signaling pathways.Mol Cell Endocrinol. 2019 May 1;487:59-65. doi: 10.1016/j.mce.2019.01.015. Epub 2019 Jan 17. Mol Cell Endocrinol. 2019. PMID: 30660699 Free PMC article. Review.
-
Relaxin inhibits cardiac fibrosis and endothelial-mesenchymal transition via the Notch pathway.Drug Des Devel Ther. 2015 Aug 11;9:4599-611. doi: 10.2147/DDDT.S85399. eCollection 2015. Drug Des Devel Ther. 2015. PMID: 26316699 Free PMC article.
-
Relaxin abrogates renal interstitial fibrosis by regulating macrophage polarization via inhibition of Toll-like receptor 4 signaling.Oncotarget. 2017 Mar 28;8(13):21044-21053. doi: 10.18632/oncotarget.15483. Oncotarget. 2017. PMID: 28416741 Free PMC article.
References
-
- Franklin TJ (1997) Therapeutic approaches to organ fibrosis. Int J Biochem Cell Biol 29: 79–89. - PubMed
-
- Becker GJ, Hewitson TD (2000) The role of tubulointerstitial injury in chronic renal failure. Curr Opin Nephrol Hypertens 9: 133–138. - PubMed
-
- Powell DW, Mifflin RC, Valentich JD, Crowe SE, Saada JI, et al. (1999) Myofibroblasts. I. Paracrine cells important in health and disease. Am J Physiol 277: C1–C9. - PubMed
-
- Weber KT (1989) Cardiac interstitium in health and disease: the fibrillar collagen network. J Am Coll Cardiol 13: 1637–1652. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

