The opposing roles of cellular inhibitor of apoptosis proteins in cancer
- PMID: 22934195
- PMCID: PMC3425795
- DOI: 10.5402/2012/928120
The opposing roles of cellular inhibitor of apoptosis proteins in cancer
Abstract
Cellular inhibitors of apoptosis proteins 1 and 2 (cIAP1/2) are members of the inhibitor of apoptosis protein (IAP) family that has been implicated in the pathology of human cancers due to their overexpression and function as blockers of cell death in various cancers. As a result, small molecule IAP antagonists have been developed and are currently under clinical evaluation for potential therapeutic use. In contrast, recent evidence has indicated a tumour-suppressing role for the cIAPs. Mutations in or loss of cIAPs have been identified as molecular lesions that contribute to constitutive activation of NF-κB in hematopoietic malignancies. These studies reveal a context-dependent role for the cIAPs wherein both their overexpression and loss may contribute to tumourigenesis.
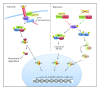
Figures
Similar articles
-
Tumor necrosis factor (TNF) signaling, but not TWEAK (TNF-like weak inducer of apoptosis)-triggered cIAP1 (cellular inhibitor of apoptosis protein 1) degradation, requires cIAP1 RING dimerization and E2 binding.J Biol Chem. 2010 Jun 4;285(23):17525-36. doi: 10.1074/jbc.M109.087635. Epub 2010 Mar 30. J Biol Chem. 2010. PMID: 20356846 Free PMC article.
-
Structure-based design and molecular profiling of Smac-mimetics selective for cellular IAPs.FEBS J. 2018 Sep;285(17):3286-3298. doi: 10.1111/febs.14616. Epub 2018 Aug 16. FEBS J. 2018. PMID: 30055105
-
Birinapant (TL32711), a bivalent SMAC mimetic, targets TRAF2-associated cIAPs, abrogates TNF-induced NF-κB activation, and is active in patient-derived xenograft models.Mol Cancer Ther. 2014 Apr;13(4):867-79. doi: 10.1158/1535-7163.MCT-13-0798. Epub 2014 Feb 21. Mol Cancer Ther. 2014. PMID: 24563541
-
Inhibitors of apoptosis proteins (IAPs) as potential molecular targets for therapy of hematological malignancies.Curr Mol Med. 2011 Nov;11(8):633-49. doi: 10.2174/156652411797536723. Curr Mol Med. 2011. PMID: 21902653 Review.
-
Molecular pathways: targeting inhibitor of apoptosis proteins in cancer--from molecular mechanism to therapeutic application.Clin Cancer Res. 2014 Jan 15;20(2):289-95. doi: 10.1158/1078-0432.CCR-13-0227. Epub 2013 Nov 22. Clin Cancer Res. 2014. PMID: 24270683 Review.
Cited by
-
50 years on and still very much alive: 'Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics'.Br J Cancer. 2023 Feb;128(3):426-431. doi: 10.1038/s41416-022-02020-0. Epub 2022 Nov 11. Br J Cancer. 2023. PMID: 36369364 Free PMC article. Review.
-
Anti-cancer IAP antagonists promote bone metastasis: a cautionary tale.J Bone Miner Metab. 2013 Sep;31(5):496-506. doi: 10.1007/s00774-013-0479-0. Epub 2013 Jun 6. J Bone Miner Metab. 2013. PMID: 23740289 Free PMC article. Review.
-
Regulation of Apoptosis by Inhibitors of Apoptosis (IAPs).Cells. 2013 Mar 14;2(1):163-87. doi: 10.3390/cells2010163. Cells. 2013. PMID: 24709650 Free PMC article.
-
Cell death in response to antimetabolites directed at ribonucleotide reductase and thymidylate synthase.Onco Targets Ther. 2015 Feb 26;8:495-507. doi: 10.2147/OTT.S79647. eCollection 2015. Onco Targets Ther. 2015. PMID: 25767396 Free PMC article.
-
The BIRC Family Genes Expression in Patients with Triple Negative Breast Cancer.Int J Mol Sci. 2021 Feb 12;22(4):1820. doi: 10.3390/ijms22041820. Int J Mol Sci. 2021. PMID: 33673050 Free PMC article. Clinical Trial.
References
-
- Salvesen GS, Duckett CS. IAP proteins: blocking the road to death’s door. Nature Reviews Molecular Cell Biology. 2002;3(6):401–410. - PubMed
-
- Hinds MG, Norton RS, Vaux DL, Day CL. Solution structure of a baculoviral inhibitor of apoptosis (IAP) repeat. Nature Structural Biology. 1999;6(7):648–651. - PubMed
-
- Eckelman BP, Salvesen GS. The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. Journal of Biological Chemistry. 2006;281(6):3254–3260. - PubMed
-
- Hu S, Yang X. Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. Journal of Biological Chemistry. 2003;278(12):10055–10060. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

