Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction
- PMID: 22932723
- PMCID: PMC3434655
- DOI: 10.1038/cddis.2012.114
Exosome-mediated shuttling of microRNA-29 regulates HIV Tat and morphine-mediated neuronal dysfunction
Abstract
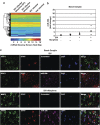
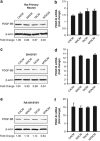
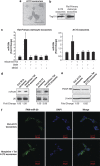
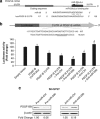
Neuronal damage is a hallmark feature of HIV-associated neurological disorders (HANDs). Opiate drug abuse accelerates the incidence and progression of HAND; however, the mechanisms underlying the potentiation of neuropathogenesis by these drugs remain elusive. Opiates such as morphine have been shown to enhance HIV transactivation protein Tat-mediated toxicity in both human neurons and neuroblastoma cells. In the present study, we demonstrate reduced expression of the tropic factor platelet-derived growth factor (PDGF)-B with a concomitant increase in miR-29b in the basal ganglia region of the brains of morphine-dependent simian immunodeficiency virus (SIV)-infected macaques compared with the SIV-infected controls. In vitro relevance of these findings was corroborated in cultures of astrocytes exposed to morphine and HIV Tat that led to increased release of miR-29b in exosomes. Subsequent treatment of neuronal SH-SY5Y cell line with exosomes from treated astrocytes resulted in decreased expression of PDGF-B, with a concomitant decrease in viability of neurons. Furthermore, it was shown that PDGF-B was a target for miR-29b as evidenced by the fact that binding of miR-29 to the 3'-untranslated region of PDGF-B mRNA resulted in its translational repression in SH-SY5Y cells. Understanding the regulation of PDGF-B expression may provide insights into the development of potential therapeutic targets for neuronal loss in HIV-1-infected opiate abusers.
Figures

Similar articles
-
Exosomal miR-9 Released from HIV Tat Stimulated Astrocytes Mediates Microglial Migration.J Neuroimmune Pharmacol. 2018 Sep;13(3):330-344. doi: 10.1007/s11481-018-9779-4. Epub 2018 Mar 1. J Neuroimmune Pharmacol. 2018. PMID: 29497921 Free PMC article.
-
A growth factor attenuates HIV-1 Tat and morphine induced damage to human neurons: implication in HIV/AIDS-drug abuse cases.PLoS One. 2011 Mar 24;6(3):e18116. doi: 10.1371/journal.pone.0018116. PLoS One. 2011. PMID: 21483469 Free PMC article.
-
A central role for glial CCR5 in directing the neuropathological interactions of HIV-1 Tat and opiates.J Neuroinflammation. 2018 Oct 10;15(1):285. doi: 10.1186/s12974-018-1320-4. J Neuroinflammation. 2018. PMID: 30305110 Free PMC article.
-
Doxycycline-inducible and astrocyte-specific HIV-1 Tat transgenic mice (iTat) as an HIV/neuroAIDS model.J Neurovirol. 2018 Apr;24(2):168-179. doi: 10.1007/s13365-017-0598-9. Epub 2017 Nov 15. J Neurovirol. 2018. PMID: 29143286 Free PMC article. Review.
-
Opiate drug use and the pathophysiology of neuroAIDS.Curr HIV Res. 2012 Jul;10(5):435-52. doi: 10.2174/157016212802138779. Curr HIV Res. 2012. PMID: 22591368 Free PMC article. Review.
Cited by
-
miRNA packaging into small extracellular vesicles and implications in pain.Pain Rep. 2024 Oct 23;9(6):e1198. doi: 10.1097/PR9.0000000000001198. eCollection 2024 Dec. Pain Rep. 2024. PMID: 39450410 Free PMC article. Review.
-
Biogenesis, physiological functions and potential applications of extracellular vesicles in substance use disorders.Cell Mol Life Sci. 2021 Jun;78(11):4849-4865. doi: 10.1007/s00018-021-03824-8. Epub 2021 Apr 5. Cell Mol Life Sci. 2021. PMID: 33821293 Free PMC article. Review.
-
HIV-1 Tat-Induced Astrocytic Extracellular Vesicle miR-7 Impairs Synaptic Architecture.J Neuroimmune Pharmacol. 2020 Sep;15(3):538-553. doi: 10.1007/s11481-019-09869-8. Epub 2019 Aug 10. J Neuroimmune Pharmacol. 2020. PMID: 31401755 Free PMC article.
-
HIV Infection Induces Extracellular Cathepsin B Uptake and Damage to Neurons.Sci Rep. 2019 May 29;9(1):8006. doi: 10.1038/s41598-019-44463-1. Sci Rep. 2019. PMID: 31142756 Free PMC article.
-
Astrocyte-derived extracellular vesicles: Neuroreparative properties and role in the pathogenesis of neurodegenerative disorders.J Control Release. 2020 Jul 10;323:225-239. doi: 10.1016/j.jconrel.2020.04.017. Epub 2020 Apr 11. J Control Release. 2020. PMID: 32289328 Free PMC article. Review.
References
-
- Kapadia F, Vlahov D, Donahoe RM, Friedland G. The role of substance abuse in HIV disease progression: reconciling differences from laboratory and epidemiologic investigations. Clin Infect Dis. 2005;41:1027–1034. - PubMed
-
- Bell JE, Arango JC, Anthony IC. Neurobiology of multiple insults: HIV-1-associated brain disorders in those who use illicit drugs. J Neuroimmune Pharmacol. 2006;1:182–191. - PubMed
-
- Salmon AY, Goren Z, Avissar Y, Soreq H. Human erythrocyte but not brain acetylcholinesterase hydrolyses heroin to morphine. Clin Exp Pharmacol Physiol. 1999;26:596–600. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

