SOCS3 deficiency promotes M1 macrophage polarization and inflammation
- PMID: 22925925
- PMCID: PMC4184888
- DOI: 10.4049/jimmunol.1201168
SOCS3 deficiency promotes M1 macrophage polarization and inflammation
Erratum in
-
Correction: SOCS3 Deficiency Promotes M1 Macrophage Polarization and Inflammation.J Immunol. 2016 Jul 1;197(1):387-9. doi: 10.4049/jimmunol.1600710. J Immunol. 2016. PMID: 27317734 No abstract available.
Abstract
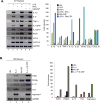
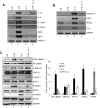
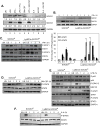
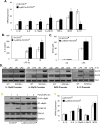
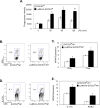
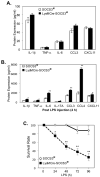
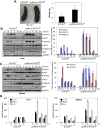
Macrophages participate in both the amplification of inflammation at the time of injury and downregulation of the inflammatory response to avoid excess tissue damage. These divergent functions of macrophages are dictated by their microenvironment, especially cytokines, which promote a spectrum of macrophage phenotypes. The M1 proinflammatory phenotype is induced by LPS, IFN-γ, and GM-CSF, and IL-4, IL-13, and M-CSF induce anti-inflammatory M2 macrophages. Suppressors of cytokine signaling (SOCS) proteins function as feedback inhibitors of the JAK/STAT signaling pathway, and they can terminate innate and adaptive immune responses. In this study, we have evaluated the influence of SOCS3 on macrophage polarization and function. Macrophages obtained from LysMCre-SOCS3(fl/fl) mice, which lack SOCS3 in myeloid lineage cells, exhibit enhanced and prolonged activation of the JAK/STAT pathway compared with macrophages from SOCS3(fl/fl) mice. Furthermore, SOCS3-deficient macrophages have higher levels of the M1 genes IL-1β, IL-6, IL-12, IL-23, and inducible NO synthase owing to enhanced transcriptional activation and chromatin modifications. SOCS3-deficient M1 macrophages also have a stronger capacity to induce Th1 and Th17 cell differentiation than M1 macrophages from SOCS3(fl/fl) mice. Lastly, LPS-induced sepsis is exacerbated in LysMCre-SOCS3(fl/fl) mice and is associated with enhanced STAT1/3 activation and increased plasma levels of M1 cytokines/chemokines such as IL-1β, TNF-α, IL-6, CCL3, CCL4, and CXCL11. These findings collectively indicate that SOCS3 is involved in repressing the M1 proinflammatory phenotype, thereby deactivating inflammatory responses in macrophages.
Figures
Similar articles
-
Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation.Proc Natl Acad Sci U S A. 2012 Mar 27;109(13):5004-9. doi: 10.1073/pnas.1117218109. Epub 2012 Mar 12. Proc Natl Acad Sci U S A. 2012. PMID: 22411837 Free PMC article.
-
Unique expression of suppressor of cytokine signaling 3 is essential for classical macrophage activation in rodents in vitro and in vivo.J Immunol. 2008 May 1;180(9):6270-8. doi: 10.4049/jimmunol.180.9.6270. J Immunol. 2008. PMID: 18424750
-
A critical role for suppressor of cytokine signalling 3 in promoting M1 macrophage activation and function in vitro and in vivo.Immunology. 2014 Jan;141(1):96-110. doi: 10.1111/imm.12173. Immunology. 2014. PMID: 24088176 Free PMC article. Clinical Trial.
-
Forkhead Box O1 Regulates Macrophage Polarization Following Staphylococcus aureus Infection: Experimental Murine Data and Review of the Literature.Clin Rev Allergy Immunol. 2016 Dec;51(3):353-369. doi: 10.1007/s12016-016-8531-1. Clin Rev Allergy Immunol. 2016. PMID: 26924010 Review.
-
Macrophage plasticity, polarization, and function in health and disease.J Cell Physiol. 2018 Sep;233(9):6425-6440. doi: 10.1002/jcp.26429. Epub 2018 Mar 1. J Cell Physiol. 2018. PMID: 29319160 Review.
Cited by
-
Prostacyclin Released by Cancer-Associated Fibroblasts Promotes Immunosuppressive and Pro-Metastatic Macrophage Polarization in the Ovarian Cancer Microenvironment.Cancers (Basel). 2022 Dec 13;14(24):6154. doi: 10.3390/cancers14246154. Cancers (Basel). 2022. PMID: 36551640 Free PMC article.
-
Jak2 Inhibitor AG490 Improved Poststroke Central and Peripheral Inflammation and Metabolic Abnormalities in a Rat Model of Ischemic Stroke.Antioxidants (Basel). 2021 Dec 7;10(12):1958. doi: 10.3390/antiox10121958. Antioxidants (Basel). 2021. PMID: 34943061 Free PMC article.
-
The gut-liver axis in sepsis: interaction mechanisms and therapeutic potential.Crit Care. 2022 Jul 13;26(1):213. doi: 10.1186/s13054-022-04090-1. Crit Care. 2022. PMID: 35831877 Free PMC article. Review.
-
Unique macrophages different from M1/M2 macrophages inhibit T cell mitogenesis while upregulating Th17 polarization.Sci Rep. 2014 Feb 20;4:4146. doi: 10.1038/srep04146. Sci Rep. 2014. PMID: 24553452 Free PMC article.
-
Along the Axis between Type 1 and Type 2 Immunity; Principles Conserved in Evolution from Fish to Mammals.Biology (Basel). 2015 Nov 17;4(4):814-59. doi: 10.3390/biology4040814. Biology (Basel). 2015. PMID: 26593954 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

