Putative glycosyltransferases and other plant Golgi apparatus proteins are revealed by LOPIT proteomics
- PMID: 22923678
- PMCID: PMC3461528
- DOI: 10.1104/pp.112.204263
Putative glycosyltransferases and other plant Golgi apparatus proteins are revealed by LOPIT proteomics
Abstract
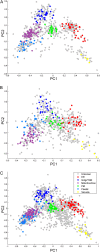
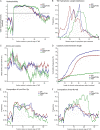
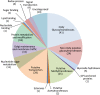
The Golgi apparatus is the central organelle in the secretory pathway and plays key roles in glycosylation, protein sorting, and secretion in plants. Enzymes involved in the biosynthesis of complex polysaccharides, glycoproteins, and glycolipids are located in this organelle, but the majority of them remain uncharacterized. Here, we studied the Arabidopsis (Arabidopsis thaliana) membrane proteome with a focus on the Golgi apparatus using localization of organelle proteins by isotope tagging. By applying multivariate data analysis to a combined data set of two new and two previously published localization of organelle proteins by isotope tagging experiments, we identified the subcellular localization of 1,110 proteins with high confidence. These include 197 Golgi apparatus proteins, 79 of which have not been localized previously by a high-confidence method, as well as the localization of 304 endoplasmic reticulum and 208 plasma membrane proteins. Comparison of the hydrophobic domains of the localized proteins showed that the single-span transmembrane domains have unique properties in each organelle. Many of the novel Golgi-localized proteins belong to uncharacterized protein families. Structure-based homology analysis identified 12 putative Golgi glycosyltransferase (GT) families that have no functionally characterized members and, therefore, are not yet assigned to a Carbohydrate-Active Enzymes database GT family. The substantial numbers of these putative GTs lead us to estimate that the true number of plant Golgi GTs might be one-third above those currently annotated. Other newly identified proteins are likely to be involved in the transport and interconversion of nucleotide sugar substrates as well as polysaccharide and protein modification.
Figures

Similar articles
-
Localization of organelle proteins by isotope tagging (LOPIT).Mol Cell Proteomics. 2004 Nov;3(11):1128-34. doi: 10.1074/mcp.T400009-MCP200. Epub 2004 Aug 4. Mol Cell Proteomics. 2004. PMID: 15295017
-
Isolation and proteomic characterization of the Arabidopsis Golgi defines functional and novel components involved in plant cell wall biosynthesis.Plant Physiol. 2012 May;159(1):12-26. doi: 10.1104/pp.111.193151. Epub 2012 Mar 19. Plant Physiol. 2012. PMID: 22430844 Free PMC article.
-
The plant glycosyltransferase clone collection for functional genomics.Plant J. 2014 Aug;79(3):517-29. doi: 10.1111/tpj.12577. Epub 2014 Jul 9. Plant J. 2014. PMID: 24905498
-
Glycosyltransferases and cell wall biosynthesis: novel players and insights.Curr Opin Plant Biol. 2004 Jun;7(3):285-95. doi: 10.1016/j.pbi.2004.03.006. Curr Opin Plant Biol. 2004. PMID: 15134749 Review.
-
Golgi-localized enzyme complexes for plant cell wall biosynthesis.Trends Plant Sci. 2013 Jan;18(1):49-58. doi: 10.1016/j.tplants.2012.07.002. Epub 2012 Aug 25. Trends Plant Sci. 2013. PMID: 22925628 Review.
Cited by
-
Proteomic dissection of the Arabidopsis Golgi and trans-Golgi network.Front Plant Sci. 2013 Jan 3;3:298. doi: 10.3389/fpls.2012.00298. eCollection 2012. Front Plant Sci. 2013. PMID: 23316206 Free PMC article.
-
Polymerization of the backbone of the pectic polysaccharide rhamnogalacturonan I.Nat Plants. 2022 Nov;8(11):1289-1303. doi: 10.1038/s41477-022-01270-3. Epub 2022 Nov 10. Nat Plants. 2022. PMID: 36357524 Free PMC article.
-
Beyond the Western front: targeted proteomics and organelle abundance profiling.Front Plant Sci. 2015 May 5;6:301. doi: 10.3389/fpls.2015.00301. eCollection 2015. Front Plant Sci. 2015. PMID: 25999968 Free PMC article.
-
Two members of the DUF579 family are responsible for arabinogalactan methylation in Arabidopsis.Plant Direct. 2019 Feb 12;3(2):e00117. doi: 10.1002/pld3.117. eCollection 2019 Feb. Plant Direct. 2019. PMID: 31245760 Free PMC article.
-
Mass-spectrometry-based spatial proteomics data analysis using pRoloc and pRolocdata.Bioinformatics. 2014 May 1;30(9):1322-4. doi: 10.1093/bioinformatics/btu013. Epub 2014 Jan 11. Bioinformatics. 2014. PMID: 24413670 Free PMC article.
References
-
- Albrecht D, Kniemeyer O, Brakhage AA, Guthke R. (2010) Missing values in gel-based proteomics. Proteomics 10: 1202–1211 - PubMed
-
- Atmodjo MA, Sakuragi Y, Zhu X, Burrell AJ, Mohanty SS, Atwood JA, III, Orlando R, Scheller HV, Mohnen D. (2011) Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan:galacturonosyltransferase complex. Proc Natl Acad Sci USA 108: 20225–20230 - PMC - PubMed
-
- Barton CJ, Tailford LE, Welchman H, Zhang Z, Gilbert HJ, Dupree P, Goubet F. (2006) Enzymatic fingerprinting of Arabidopsis pectic polysaccharides using polysaccharide analysis by carbohydrate gel electrophoresis (PACE). Planta 224: 163–174 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

