Molecular mechanics of cardiac myosin-binding protein C in native thick filaments
- PMID: 22923435
- PMCID: PMC3561468
- DOI: 10.1126/science.1223602
Molecular mechanics of cardiac myosin-binding protein C in native thick filaments
Abstract
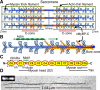
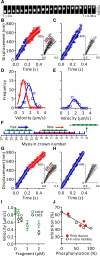
The heart's pumping capacity results from highly regulated interactions of actomyosin molecular motors. Mutations in the gene for a potential regulator of these motors, cardiac myosin-binding protein C (cMyBP-C), cause hypertrophic cardiomyopathy. However, cMyBP-C's ability to modulate cardiac contractility is not well understood. Using single-particle fluorescence imaging techniques, transgenic protein expression, proteomics, and modeling, we found that cMyBP-C slowed actomyosin motion generation in native cardiac thick filaments. This mechanical effect was localized to where cMyBP-C resides within the thick filament (i.e., the C-zones) and was modulated by phosphorylation and site-specific proteolytic degradation. These results provide molecular insight into why cMyBP-C should be considered a member of a tripartite complex with actin and myosin that allows fine tuning of cardiac muscle contraction.
Figures
Comment in
-
Cell biology. Heart brakes.Science. 2012 Sep 7;337(6099):1182-3. doi: 10.1126/science.1227943. Science. 2012. PMID: 22955824 No abstract available.
Similar articles
-
Molecular modulation of actomyosin function by cardiac myosin-binding protein C.Pflugers Arch. 2014 Mar;466(3):439-44. doi: 10.1007/s00424-013-1433-7. Epub 2014 Jan 10. Pflugers Arch. 2014. PMID: 24407948 Free PMC article. Review.
-
Site-specific phosphorylation of myosin binding protein-C coordinates thin and thick filament activation in cardiac muscle.Proc Natl Acad Sci U S A. 2019 Jul 30;116(31):15485-15494. doi: 10.1073/pnas.1903033116. Epub 2019 Jul 15. Proc Natl Acad Sci U S A. 2019. PMID: 31308242 Free PMC article.
-
In vivo definition of cardiac myosin-binding protein C's critical interactions with myosin.Pflugers Arch. 2016 Oct;468(10):1685-95. doi: 10.1007/s00424-016-1873-y. Epub 2016 Aug 27. Pflugers Arch. 2016. PMID: 27568194 Free PMC article.
-
Multiple forms of cardiac myosin-binding protein C exist and can regulate thick filament stability.J Gen Physiol. 2007 May;129(5):419-28. doi: 10.1085/jgp.200609714. J Gen Physiol. 2007. PMID: 17470661 Free PMC article.
-
Post-translational control of cardiac hemodynamics through myosin binding protein C.Pflugers Arch. 2014 Feb;466(2):231-6. doi: 10.1007/s00424-013-1377-y. Epub 2013 Oct 22. Pflugers Arch. 2014. PMID: 24145982 Free PMC article. Review.
Cited by
-
A post-MI power struggle: adaptations in cardiac power occur at the sarcomere level alongside MyBP-C and RLC phosphorylation.Am J Physiol Heart Circ Physiol. 2016 Aug 1;311(2):H465-75. doi: 10.1152/ajpheart.00899.2015. Epub 2016 May 27. Am J Physiol Heart Circ Physiol. 2016. PMID: 27233767 Free PMC article.
-
Myosin-binding protein H-like regulates myosin-binding protein distribution and function in atrial cardiomyocytes.Proc Natl Acad Sci U S A. 2023 Dec 19;120(51):e2314920120. doi: 10.1073/pnas.2314920120. Epub 2023 Dec 13. Proc Natl Acad Sci U S A. 2023. PMID: 38091294 Free PMC article.
-
Cardiac myosin binding protein-C phosphorylation regulates the super-relaxed state of myosin.Proc Natl Acad Sci U S A. 2019 Jun 11;116(24):11731-11736. doi: 10.1073/pnas.1821660116. Epub 2019 May 29. Proc Natl Acad Sci U S A. 2019. PMID: 31142654 Free PMC article.
-
Molecular modulation of actomyosin function by cardiac myosin-binding protein C.Pflugers Arch. 2014 Mar;466(3):439-44. doi: 10.1007/s00424-013-1433-7. Epub 2014 Jan 10. Pflugers Arch. 2014. PMID: 24407948 Free PMC article. Review.
-
Assessing Cardiac Contractility From Single Molecules to Whole Hearts.JACC Basic Transl Sci. 2023 Oct 11;9(3):414-439. doi: 10.1016/j.jacbts.2023.07.013. eCollection 2024 Mar. JACC Basic Transl Sci. 2023. PMID: 38559627 Free PMC article. Review.
References
-
- Winegrad S. Cardiac myosin binding protein C. Circ Res. 1999;84:1117. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

