Tau promotes neurodegeneration via DRP1 mislocalization in vivo
- PMID: 22920254
- PMCID: PMC3428596
- DOI: 10.1016/j.neuron.2012.06.026
Tau promotes neurodegeneration via DRP1 mislocalization in vivo
Abstract
Mitochondrial abnormalities have been documented in Alzheimer's disease and related neurodegenerative disorders, but the causal relationship between mitochondrial changes and neurodegeneration, and the specific mechanisms promoting mitochondrial dysfunction, are unclear. Here, we find that expression of human tau results in elongation of mitochondria in both Drosophila and mouse neurons. Elongation is accompanied by mitochondrial dysfunction and cell cycle-mediated cell death, which can be rescued in vivo by genetically restoring the proper balance of mitochondrial fission and fusion. We have previously demonstrated that stabilization of actin by tau is critical for neurotoxicity of the protein. Here, we demonstrate a conserved role for actin and myosin in regulating mitochondrial fission and show that excess actin stabilization inhibits association of the fission protein DRP1 with mitochondria, leading to mitochondrial elongation and subsequent neurotoxicity. Our results thus identify actin-mediated disruption of mitochondrial dynamics as a direct mechanism of tau toxicity in neurons in vivo.
Copyright © 2012 Elsevier Inc. All rights reserved.
Figures
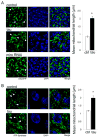
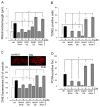
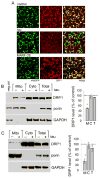
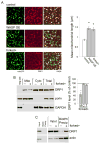
Similar articles
-
Abnormal interaction between the mitochondrial fission protein Drp1 and hyperphosphorylated tau in Alzheimer's disease neurons: implications for mitochondrial dysfunction and neuronal damage.Hum Mol Genet. 2012 Jun 1;21(11):2538-47. doi: 10.1093/hmg/dds072. Epub 2012 Feb 24. Hum Mol Genet. 2012. PMID: 22367970 Free PMC article.
-
Impaired balance of mitochondrial fission and fusion in Alzheimer's disease.J Neurosci. 2009 Jul 15;29(28):9090-103. doi: 10.1523/JNEUROSCI.1357-09.2009. J Neurosci. 2009. PMID: 19605646 Free PMC article.
-
Inner-membrane proteins PMI/TMEM11 regulate mitochondrial morphogenesis independently of the DRP1/MFN fission/fusion pathways.EMBO Rep. 2011 Mar;12(3):223-30. doi: 10.1038/embor.2010.214. Epub 2011 Jan 28. EMBO Rep. 2011. PMID: 21274005 Free PMC article.
-
The role of Drp1 adaptor proteins MiD49 and MiD51 in mitochondrial fission: implications for human disease.Clin Sci (Lond). 2016 Nov 1;130(21):1861-74. doi: 10.1042/CS20160030. Clin Sci (Lond). 2016. PMID: 27660309 Review.
-
S-nitrosylation of Drp1 links excessive mitochondrial fission to neuronal injury in neurodegeneration.Mitochondrion. 2010 Aug;10(5):573-8. doi: 10.1016/j.mito.2010.04.007. Epub 2010 May 4. Mitochondrion. 2010. PMID: 20447471 Free PMC article. Review.
Cited by
-
Transient assembly of F-actin on the outer mitochondrial membrane contributes to mitochondrial fission.J Cell Biol. 2015 Jan 5;208(1):109-23. doi: 10.1083/jcb.201404050. Epub 2014 Dec 29. J Cell Biol. 2015. PMID: 25547155 Free PMC article.
-
Modeling the complex pathology of Alzheimer's disease in Drosophila.Exp Neurol. 2015 Dec;274(Pt A):58-71. doi: 10.1016/j.expneurol.2015.05.013. Epub 2015 May 27. Exp Neurol. 2015. PMID: 26024860 Free PMC article. Review.
-
The importance of tau phosphorylation for neurodegenerative diseases.Front Neurol. 2013 Jul 1;4:83. doi: 10.3389/fneur.2013.00083. eCollection 2013. Front Neurol. 2013. PMID: 23847585 Free PMC article.
-
Simple model systems reveal conserved mechanisms of Alzheimer's disease and related tauopathies.Mol Neurodegener. 2023 Nov 10;18(1):82. doi: 10.1186/s13024-023-00664-x. Mol Neurodegener. 2023. PMID: 37950311 Free PMC article. Review.
-
Changes in Drp1 Function and Mitochondrial Morphology Are Associated with the α-Synuclein Pathology in a Transgenic Mouse Model of Parkinson's Disease.Cells. 2021 Apr 13;10(4):885. doi: 10.3390/cells10040885. Cells. 2021. PMID: 33924585 Free PMC article.
References
-
- Ahlijanian MK, Barrezueta NX, Williams RD, Jakowski A, Kowsz KP, McCarthy S, Coskran T, Carlo A, Seymour PA, Burkhardt JE, Nelson RB, McNeish JD. Hyperphosphorylated tau and neurofilament and cytoskeletal disruptions in mice overexpressing human p25, an activator of cdk5. Proc Natl Acad Sci U S A. 2000;97:2910–2915. - PMC - PubMed
-
- Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B. OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet. 2000;26:211–215. - PubMed
-
- Berger S, Schafer G, Kesper DA, Holz A, Eriksson T, Palmer RH, Beck L, Klambt C, Renkawitz-Pohl R, Onel SF. WASP and SCAR have distinct roles in activating the Arp2/3 complex during myoblast fusion. J Cell Sci. 2008;121:1303–1313. - PubMed
-
- Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–15. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

