Antifibrotic activity of sorafenib in experimental hepatic fibrosis: refinement of inhibitory targets, dosing, and window of efficacy in vivo
- PMID: 22918681
- PMCID: PMC3543488
- DOI: 10.1007/s10620-012-2325-y
Antifibrotic activity of sorafenib in experimental hepatic fibrosis: refinement of inhibitory targets, dosing, and window of efficacy in vivo
Abstract
Background: Sorafenib, which is approved for treatment of HCC, has also shown promising antifibrotic activity, and therefore refinement of its dosing requirements and window of efficacy are important goals prior to antifibrotic clinical trials.
Aim: The purpose of this study was to determine the minimal effective dose and optimal timing of sorafenib therapy in cultured human stellate cells and in rats with experimental hepatic fibrosis.
Methods: Effects of sorafenib were assessed in a human stellate cell line (LX-2). In vivo, rats were treated for 8 weeks with TAA three times per week (150 mg/kg IP), and with either PBS or sorafenib administered daily at doses of 1.25, 5 or 7 mg/kg/day gavage either at the beginning of TAA administration for 8 weeks, during weeks 4-8, or from weeks 8-12.
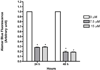
Results: Sorafenib treatment significantly inhibited LX-2 proliferation by >75% (7.5 or 15 μM). Treatment with 7.5-μM sorafenib for 12 h markedly inhibited expression of TGFβ1, TIMP-1, collagen I, and MMP2 mRNAs, but not of β-PDGFR or type I TGFβR. In vivo, sorafenib significantly inhibited liver fibrosis when started concurrently with TAA and during weeks 4-8 with TAA. In contrast, there was no significant effect of sorafenib on fibrogenic gene expression or fibrosis when begun after cirrhosis was already established.
Conclusion: Sorafenib is anti-proliferative and antifibrotic towards human HSCs in culture, and is a potent antifibrotic agent in TAA-induced hepatic fibrosis in rats. The drug is effective at relatively low doses at the early stage of liver fibrosis, but is not effective when cirrhosis is already established.
Figures


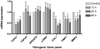

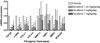





Similar articles
-
Sorafenib and its derivative SC-1 exhibit antifibrotic effects through signal transducer and activator of transcription 3 inhibition.Proc Natl Acad Sci U S A. 2015 Jun 9;112(23):7243-8. doi: 10.1073/pnas.1507499112. Epub 2015 May 26. Proc Natl Acad Sci U S A. 2015. PMID: 26039995 Free PMC article.
-
Sorafenib and fluvastatin synergistically alleviate hepatic fibrosis via inhibiting the TGFβ1/Smad3 pathway.Dig Liver Dis. 2018 Apr;50(4):381-388. doi: 10.1016/j.dld.2017.12.015. Epub 2017 Dec 28. Dig Liver Dis. 2018. PMID: 29373239
-
Antifibrotic effects of magnesium lithospermate B on hepatic stellate cells and thioacetamide-induced cirrhotic rats.Exp Mol Med. 2011 Jun 30;43(6):341-9. doi: 10.3858/emm.2011.43.6.037. Exp Mol Med. 2011. PMID: 21499011 Free PMC article.
-
Sorafenib: A potential therapeutic drug for hepatic fibrosis and its outcomes.Biomed Pharmacother. 2017 Apr;88:459-468. doi: 10.1016/j.biopha.2017.01.107. Epub 2017 Jan 22. Biomed Pharmacother. 2017. PMID: 28122312 Review.
-
Biology, Epidemiology, Clinical Aspects of Hepatocellular Carcinoma and the Role of Sorafenib.Curr Drug Targets. 2016;17(7):783-99. doi: 10.2174/1389450117666151209120831. Curr Drug Targets. 2016. PMID: 26648069 Review.
Cited by
-
The influence of astragalus polysaccharide and β-elemene on LX-2 cell growth, apoptosis and activation.BMC Gastroenterol. 2014 Dec 31;14:224. doi: 10.1186/s12876-014-0224-8. BMC Gastroenterol. 2014. PMID: 25551689 Free PMC article.
-
CCL2-dependent infiltrating macrophages promote angiogenesis in progressive liver fibrosis.Gut. 2014 Dec;63(12):1960-1971. doi: 10.1136/gutjnl-2013-306294. Epub 2014 Feb 21. Gut. 2014. PMID: 24561613 Free PMC article.
-
Repositioning of a novel GABA-B receptor agonist, AZD3355 (Lesogaberan), for the treatment of non-alcoholic steatohepatitis.Sci Rep. 2021 Oct 21;11(1):20827. doi: 10.1038/s41598-021-99008-2. Sci Rep. 2021. PMID: 34675338 Free PMC article.
-
Levistilide A inhibits angiogenesis in liver fibrosis via vascular endothelial growth factor signaling pathway.Exp Biol Med (Maywood). 2017 May;242(9):974-985. doi: 10.1177/1535370217701005. Epub 2017 Mar 22. Exp Biol Med (Maywood). 2017. PMID: 28440736 Free PMC article.
-
Hepatic stellate cells as key target in liver fibrosis.Adv Drug Deliv Rev. 2017 Nov 1;121:27-42. doi: 10.1016/j.addr.2017.05.007. Epub 2017 May 12. Adv Drug Deliv Rev. 2017. PMID: 28506744 Free PMC article. Review.
References
-
- Pinzani M, Marra F. Cytokine receptors and signaling in hepatic stellate cells. Seminars in liver disease. 2001;21(3):397–416. - PubMed
-
- Borkham-Kamphorst E, et al. Pro-fibrogenic potential of PDGF-D in liver fibrosis. Journal of hepatology. 2007;46(6):1064–1074. - PubMed
-
- Wilhelm SM, et al. Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Molecular cancer therapeutics. 2008;7(10):3129–3140. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

