Porcine circovirus type 2 induces autophagy via the AMPK/ERK/TSC2/mTOR signaling pathway in PK-15 cells
- PMID: 22915817
- PMCID: PMC3486458
- DOI: 10.1128/JVI.01434-12
Porcine circovirus type 2 induces autophagy via the AMPK/ERK/TSC2/mTOR signaling pathway in PK-15 cells
Abstract
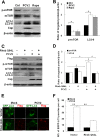
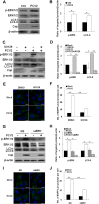
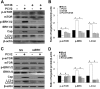
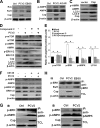
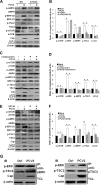
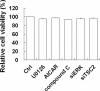
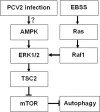
Porcine circovirus type 2 (PCV2) uses autophagy machinery to enhance its replication in PK-15 cells. However, the underlying mechanisms are unknown. By the use of specific inhibitors, RNA interference, and coimmunoprecipitation, we show that PCV2 induces autophagy in PK-15 cells through a pathway involving the kinases AMP-activated protein kinase (AMPK) and extracellular signal-regulated kinase 1/2 (ERK1/2), the tumor suppressor protein TSC2, and the mammalian target of rapamycin (mTOR). AMPK and ERK1/2 positively regulate autophagy through negative control of the mTOR pathway by phosphorylating TSC2 in PCV2-infected PK-15 cells. Thus, PCV2 might induce autophagy via the AMPK/ERK/TSC2/mTOR signaling pathway in the host cells, representing a pivotal mechanism for PCV2 pathogenesis.
Figures
Similar articles
-
Porcine Circovirus Type 2 Activates CaMMKβ to Initiate Autophagy in PK-15 Cells by Increasing Cytosolic Calcium.Viruses. 2016 May 20;8(5):135. doi: 10.3390/v8050135. Viruses. 2016. PMID: 27213427 Free PMC article.
-
Cellular p32 Is a Critical Regulator of Porcine Circovirus Type 2 Nuclear Egress.J Virol. 2019 Nov 13;93(23):e00979-19. doi: 10.1128/JVI.00979-19. Print 2019 Dec 1. J Virol. 2019. PMID: 31511386 Free PMC article.
-
Taurine attenuates OTA-promoted PCV2 replication through blocking ROS-dependent autophagy via inhibiting AMPK/mTOR signaling pathway.Chem Biol Interact. 2018 Dec 25;296:220-228. doi: 10.1016/j.cbi.2018.10.005. Epub 2018 Oct 14. Chem Biol Interact. 2018. PMID: 30332612
-
LKB1 and AMP-activated protein kinase control of mTOR signalling and growth.Acta Physiol (Oxf). 2009 May;196(1):65-80. doi: 10.1111/j.1748-1716.2009.01972.x. Epub 2009 Feb 19. Acta Physiol (Oxf). 2009. PMID: 19245654 Free PMC article. Review.
-
Role of AMP-activated protein kinase in cancer therapy.Arch Pharm (Weinheim). 2014 Jul;347(7):457-68. doi: 10.1002/ardp.201300402. Epub 2014 Mar 28. Arch Pharm (Weinheim). 2014. PMID: 24677093 Review.
Cited by
-
Chansu inhibits the expression of cortactin in colon cancer cell lines in vitro and in vivo.BMC Complement Altern Med. 2015 Jul 2;15:207. doi: 10.1186/s12906-015-0723-3. BMC Complement Altern Med. 2015. PMID: 26134506 Free PMC article.
-
Revisiting Porcine Circovirus Infection: Recent Insights and Its Significance in the Piggery Sector.Vaccines (Basel). 2023 Jul 31;11(8):1308. doi: 10.3390/vaccines11081308. Vaccines (Basel). 2023. PMID: 37631876 Free PMC article. Review.
-
Dissection and integration of the autophagy signaling network initiated by bluetongue virus infection: crucial candidates ERK1/2, Akt and AMPK.Sci Rep. 2016 Mar 15;6:23130. doi: 10.1038/srep23130. Sci Rep. 2016. PMID: 26976147 Free PMC article.
-
Glutamine starvation enhances PCV2 replication via the phosphorylation of p38 MAPK, as promoted by reducing glutathione levels.Vet Res. 2015 Mar 18;46(1):32. doi: 10.1186/s13567-015-0168-1. Vet Res. 2015. PMID: 25879878 Free PMC article.
-
The Multi-Faceted Role of Autophagy During Animal Virus Infection.Front Cell Infect Microbiol. 2022 Mar 25;12:858953. doi: 10.3389/fcimb.2022.858953. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35402295 Free PMC article. Review.
References
-
- Beach NM, Meng XJ. 2012. Efficacy and future prospects of commercially available and experimental vaccines against porcine circovirus type 2 (PCV2). Virus Res. 164:33–42 - PubMed
-
- Bolin SR, Stoffregen WC, Nayar GP, Hamel AL. 2001. Postweaning multisystemic wasting syndrome induced after experimental inoculation of cesarean-derived, colostrum-deprived piglets with type 2 porcine circovirus. J. Vet. Diagn. Invest. 13:185–194 - PubMed
-
- Botti J, Djavaheri-Mergny M, Pilatte Y, Codogno P. 2006. Autophagy signaling and the cogwheels of cancer. Autophagy 2:67–73 - PubMed
-
- Cagnol S, Chambard JC. 2010. ERK and cell death: mechanisms of ERK-induced cell death—apoptosis, autophagy, and senescence. FEBS J. 277:2–21 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

