Cytomegalovirus CC chemokine promotes immune cell migration
- PMID: 22915808
- PMCID: PMC3486311
- DOI: 10.1128/JVI.00452-12
Cytomegalovirus CC chemokine promotes immune cell migration
Abstract
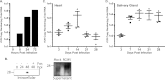
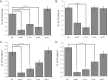
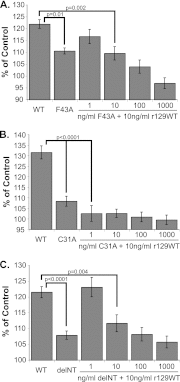
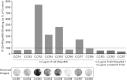
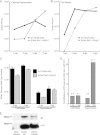
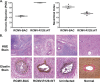
Cytomegaloviruses manipulate the host chemokine/receptor axis by altering cellular chemokine expression and by encoding multiple chemokines and chemokine receptors. Similar to human cytomegalovirus (HCMV), rat cytomegalovirus (RCMV) encodes multiple CC chemokine-analogous proteins, including r129 (HCMV UL128 homologue) and r131 (HCMV UL130 and MCMV m129/130 homologues). Although these proteins play a role in CMV entry, their function as chemotactic cytokines remains unknown. In the current study, we examined the role of the RCMV chemokine r129 in promoting cellular migration and in accelerating transplant vascular sclerosis (TVS) in our rat heart transplant model. We determined that r129 protein is released into culture supernatants of infected cells and is expressed with late viral gene kinetics during RCMV infection and highly expressed in heart and salivary glands during in vivo rat infections. Using the recombinant r129 protein, we demonstrated that r129 induces migration of lymphocytes isolated from rat peripheral blood, spleen, and bone marrow and from a rat macrophage cell line. Using antibody-mediated cell sorting of rat splenocytes, we demonstrated that r129 induces migration of naïve/central memory CD4(+) T cells. Through ligand-binding assays, we determined that r129 binds rat CC chemokine receptors CCR3, CCR4, CCR5, and CCR7. In addition, mutational analyses identified functional domains of r129 resulting in recombinant proteins that fail to induce migration (r129-ΔNT and -C31A) or alter the chemotactic ability of the chemokine (r129-F43A). Two of the mutant proteins (r129-C31A and -ΔNT) also act as dominant negatives by inhibiting migration induced by wild-type r129. Furthermore, infection of rat heart transplant recipients with RCMV containing the r129-ΔNT mutation prevented CMV-induced acceleration of TVS. Together our findings indicate that RCMV r129 is highly chemotactic, which has important implications during RCMV infection and reactivation and acceleration of TVS.
Figures

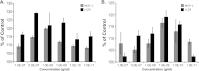


Similar articles
-
The r131 gene of rat cytomegalovirus encodes a proinflammatory CC chemokine homolog which is essential for the production of infectious virus in the salivary glands.Virus Genes. 2004 Aug;29(1):43-61. doi: 10.1023/B:VIRU.0000032788.53592.7c. Virus Genes. 2004. PMID: 15215683
-
Rat Cytomegalovirus Virion-Associated Proteins R131 and R129 Are Necessary for Infection of Macrophages and Dendritic Cells.Pathogens. 2020 Nov 19;9(11):963. doi: 10.3390/pathogens9110963. Pathogens. 2020. PMID: 33228102 Free PMC article.
-
Fatal attraction: cytomegalovirus-encoded chemokine homologs.Curr Top Microbiol Immunol. 2002;269:235-56. doi: 10.1007/978-3-642-59421-2_14. Curr Top Microbiol Immunol. 2002. PMID: 12224512
-
Mechanisms of cytomegalovirus-accelerated vascular disease: induction of paracrine factors that promote angiogenesis and wound healing.Curr Top Microbiol Immunol. 2008;325:397-415. doi: 10.1007/978-3-540-77349-8_22. Curr Top Microbiol Immunol. 2008. PMID: 18637518 Free PMC article. Review.
-
The HCMV chemokine receptor US28 is a potential target in vascular disease.Curr Drug Targets Infect Disord. 2001 Aug;1(2):151-8. doi: 10.2174/1568005014606080. Curr Drug Targets Infect Disord. 2001. PMID: 12455411 Review.
Cited by
-
Investigation of the Involvement of HHV-6 Encoded Viral Chemokine Receptors in Autoimmune Thyroiditis Development.Microbiol Spectr. 2022 Jun 29;10(3):e0236921. doi: 10.1128/spectrum.02369-21. Epub 2022 May 23. Microbiol Spectr. 2022. PMID: 35604160 Free PMC article.
-
The viral chemokine MCK-2 of murine cytomegalovirus promotes infection as part of a gH/gL/MCK-2 complex.PLoS Pathog. 2013;9(7):e1003493. doi: 10.1371/journal.ppat.1003493. Epub 2013 Jul 25. PLoS Pathog. 2013. PMID: 23935483 Free PMC article.
-
Cytomegalovirus UL128 homolog mutants that form a pentameric complex produce virus with impaired epithelial and trophoblast cell tropism and altered pathogenicity in the guinea pig.Virology. 2017 Sep;509:205-221. doi: 10.1016/j.virol.2017.06.008. Epub 2017 Jun 23. Virology. 2017. PMID: 28651121 Free PMC article.
-
Stuck in the middle: structural insights into the role of the gH/gL heterodimer in herpesvirus entry.Curr Opin Virol. 2013 Feb;3(1):13-9. doi: 10.1016/j.coviro.2012.10.005. Epub 2012 Oct 26. Curr Opin Virol. 2013. PMID: 23107819 Free PMC article. Review.
-
Murine Cytomegalovirus Infection of Melanoma Lesions Delays Tumor Growth by Recruiting and Repolarizing Monocytic Phagocytes in the Tumor.J Virol. 2019 Sep 30;93(20):e00533-19. doi: 10.1128/JVI.00533-19. Print 2019 Oct 15. J Virol. 2019. PMID: 31375579 Free PMC article.
References
-
- Akter P, et al. 2003. Two novel spliced genes in human cytomegalovirus. J. Gen. Virol. 84:1117–1122 - PubMed
-
- Armstrong AT, Strauch AR, Starling RC, Sedmak DD, Orosz CG. 1997. Morphometric analysis of neointimal formation in murine cardiac allografts. Transplantation 63:941–947 - PubMed
-
- Boomker JM, de Leij LF, The TH, Harmsen MC. 2005. Viral chemokine-modulatory proteins: tools and targets. Cytokine Growth Factor Rev. 16:91–103 - PubMed
-
- Bruggeman CA, Meijer H, Bosman F, van Boven CP. 1985. Biology of rat cytomegalovirus infection. Intervirology 24:1–9 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

