Late-onset Parkinsonism in NFκB/c-Rel-deficient mice
- PMID: 22915735
- PMCID: PMC3437025
- DOI: 10.1093/brain/aws193
Late-onset Parkinsonism in NFκB/c-Rel-deficient mice
Abstract
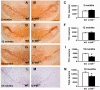
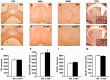
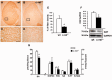
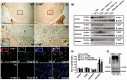
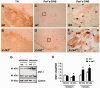
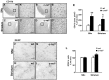
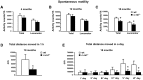
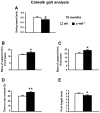
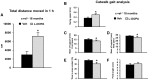
Activation of the nuclear factor κB/c-Rel can increase neuronal resilience to pathological noxae by regulating the expression of pro-survival manganese superoxide dismutase (MnSOD, now known as SOD2) and Bcl-xL genes. We show here that c-Rel-deficient (c-rel(-/-)) mice developed a Parkinson's disease-like neuropathology with ageing. At 18 months of age, c-rel(-/-) mice exhibited a significant loss of dopaminergic neurons in the substantia nigra pars compacta, as assessed by tyrosine hydroxylase-immunoreactivity and Nissl staining. Nigral degeneration was accompanied by a significant loss of dopaminergic terminals and a significant reduction of dopamine and homovanillic acid levels in the striatum. Mice deficient of the c-Rel factor exhibited a marked immunoreactivity for fibrillary α-synuclein in the substantia nigra pars compacta as well as increased expression of divalent metal transporter 1 (DMT1) and iron staining in both the substantia nigra pars compacta and striatum. Aged c-rel(-/-) mouse brain were characterized by increased microglial reactivity in the basal ganglia, but no astrocytic reaction. In addition, c-rel(-/-) mice showed age-dependent deficits in locomotor and total activity and various gait-related deficits during a catwalk analysis that were reminiscent of bradykinesia and muscle rigidity. Both locomotor and gait-related deficits recovered in c-rel(-/-) mice treated with l-3,4-dihydroxyphenylalanine. These data suggest that c-Rel may act as a regulator of the substantia nigra pars compacta resilience to ageing and that aged c-rel(-/-) mice may be a suitable model of Parkinson's disease.
Figures
Similar articles
-
AAV1/2-induced overexpression of A53T-α-synuclein in the substantia nigra results in degeneration of the nigrostriatal system with Lewy-like pathology and motor impairment: a new mouse model for Parkinson's disease.Acta Neuropathol Commun. 2017 Feb 1;5(1):11. doi: 10.1186/s40478-017-0416-x. Acta Neuropathol Commun. 2017. PMID: 28143577 Free PMC article.
-
Accumulation of mitochondrial DNA deletions within dopaminergic neurons triggers neuroprotective mechanisms.Brain. 2013 Aug;136(Pt 8):2369-78. doi: 10.1093/brain/awt196. Brain. 2013. PMID: 23884809
-
Deficits in dopaminergic transmission precede neuron loss and dysfunction in a new Parkinson model.Proc Natl Acad Sci U S A. 2013 Oct 15;110(42):E4016-25. doi: 10.1073/pnas.1309143110. Epub 2013 Sep 30. Proc Natl Acad Sci U S A. 2013. PMID: 24082145 Free PMC article.
-
Age and Parkinson's disease-related neuronal death in the substantia nigra pars compacta.J Neural Transm Suppl. 2009;(73):203-13. doi: 10.1007/978-3-211-92660-4_16. J Neural Transm Suppl. 2009. PMID: 20411779 Review.
-
M30, a brain permeable multitarget neurorestorative drug in post nigrostriatal dopamine neuron lesion of parkinsonism animal models.Parkinsonism Relat Disord. 2012 Jan;18 Suppl 1:S151-4. doi: 10.1016/S1353-8020(11)70047-5. Parkinsonism Relat Disord. 2012. PMID: 22166418 Review.
Cited by
-
NF-kappaB Signaling Pathways in Neurological Inflammation: A Mini Review.Front Mol Neurosci. 2015 Dec 18;8:77. doi: 10.3389/fnmol.2015.00077. eCollection 2015. Front Mol Neurosci. 2015. PMID: 26733801 Free PMC article. Review.
-
Overexpression of the ubiquitin-editing enzyme A20 in the brain lesions of Multiple Sclerosis patients: moving from systemic to central nervous system inflammation.Brain Pathol. 2021 Mar;31(2):283-296. doi: 10.1111/bpa.12906. Epub 2020 Nov 18. Brain Pathol. 2021. PMID: 33051914 Free PMC article.
-
Characterization of exogenous αSN response genes and their relation to Parkinson's disease using network analyses.Front Pharmacol. 2022 Sep 30;13:966760. doi: 10.3389/fphar.2022.966760. eCollection 2022. Front Pharmacol. 2022. PMID: 36249814 Free PMC article.
-
Microglial phenotypes in Parkinson's disease and animal models of the disease.Prog Neurobiol. 2017 Aug;155:57-75. doi: 10.1016/j.pneurobio.2016.04.006. Epub 2016 Apr 20. Prog Neurobiol. 2017. PMID: 27107797 Free PMC article. Review.
-
NF-κB-Mediated Neuroinflammation in Parkinson's Disease and Potential Therapeutic Effect of Polyphenols.Neurotox Res. 2020 Mar;37(3):491-507. doi: 10.1007/s12640-019-00147-2. Epub 2019 Dec 10. Neurotox Res. 2020. PMID: 31823227 Review.
References
-
- Arbuthnott GW, Wickens J. Space, time and dopamine. Trends Neurosci. 2007;30:62–9. - PubMed
-
- Bellucci A, Zaltieri M, Navarria L, Grigoletto J, Missale C, Spano P. From α-synuclein to synaptic dysfunctions: new insights into the pathophysiology of Parkinson’s disease. Brain Res. 2012 In press. - PubMed
-
- Bernard D, Quatannens B, Begue A, Vandenbunder B, Abbadie C. Antiproliferative and antiapoptotic effects of crel may occur within the same cells via the up-regulation of manganese superoxide dismutase. Cancer Res. 2001;61:2656–64. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

